Telomere biology disorders
- PMID: 34050178
- PMCID: PMC8163767
- DOI: 10.1038/s41525-021-00198-5
Telomere biology disorders
Abstract
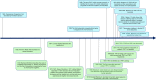
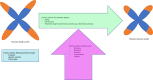
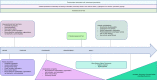
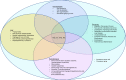
Telomere biology disorders (TBD) are a heterogeneous group of diseases arising from germline mutations affecting genes involved in telomere maintenance. Telomeres are DNA-protein structures at chromosome ends that maintain chromosome stability; their length affects cell replicative potential and senescence. A constellation of bone marrow failure, pulmonary fibrosis, liver cirrhosis and premature greying is suggestive, however incomplete penetrance results in highly variable manifestations, with idiopathic pulmonary fibrosis as the most common presentation. Currently, the true extent of TBD burden is unknown as there is no established diagnostic criteria and the disorder often is unrecognised and underdiagnosed. There is no gold standard for measuring telomere length and not all TBD-related mutations have been identified. There is no specific cure and the only treatment is organ transplantation, which has poor outcomes. This review summarises the current literature and discusses gaps in understanding and areas of need in managing TBD.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

