Mitochondrial targeted meganuclease as a platform to eliminate mutant mtDNA in vivo
- PMID: 34050192
- PMCID: PMC8163834
- DOI: 10.1038/s41467-021-23561-7
Mitochondrial targeted meganuclease as a platform to eliminate mutant mtDNA in vivo
Abstract
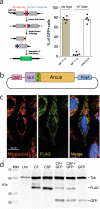
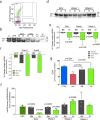
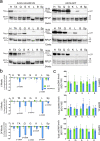
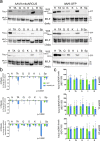
Diseases caused by heteroplasmic mitochondrial DNA mutations have no effective treatment or cure. In recent years, DNA editing enzymes were tested as tools to eliminate mutant mtDNA in heteroplasmic cells and tissues. Mitochondrial-targeted restriction endonucleases, ZFNs, and TALENs have been successful in shifting mtDNA heteroplasmy, but they all have drawbacks as gene therapy reagents, including: large size, heterodimeric nature, inability to distinguish single base changes, or low flexibility and effectiveness. Here we report the adaptation of a gene editing platform based on the I-CreI meganuclease known as ARCUS®. These mitochondrial-targeted meganucleases (mitoARCUS) have a relatively small size, are monomeric, and can recognize sequences differing by as little as one base pair. We show the development of a mitoARCUS specific for the mouse m.5024C>T mutation in the mt-tRNAAla gene and its delivery to mice intravenously using AAV9 as a vector. Liver and skeletal muscle show robust elimination of mutant mtDNA with concomitant restoration of mt-tRNAAla levels. We conclude that mitoARCUS is a potential powerful tool for the elimination of mutant mtDNA.
Conflict of interest statement
The authors declare the following competing interests: W.S., J.S., G.T., and D.J. work at Precision BioSciences, which is researching and developing ARCUS technology, and may research, develop, and commercialize mitochondria-targeted ARCUS nucleases in the future. C.T.M., D.J., G.T., and W.S. are named in a joint patent application (Patent applicant- Precision BioSciences, Inc. and the University of Miami; application number: 63/178,269; status: pending) for mitochondria-targeted ARCUS nucleases with Precision BioSciences. Specific aspect of manuscript covered in patent application: ARCUS nuclease sequences and compositions for targeting mitochondria, including delivery vectors; Methods of modifying cells, Treating mitochondrial conditions in vivo using the disclosed ARCUS nucleases. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

