Aurora kinase A, a synthetic lethal target for precision cancer medicine
- PMID: 34050264
- PMCID: PMC8178373
- DOI: 10.1038/s12276-021-00635-6
Aurora kinase A, a synthetic lethal target for precision cancer medicine
Abstract
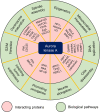
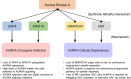
Recent advances in high-throughput sequencing technologies and data science have facilitated the development of precision medicine to treat cancer patients. Synthetic lethality is one of the core methodologies employed in precision cancer medicine. Synthetic lethality describes the phenomenon of the interplay between two genes in which deficiency of a single gene does not abolish cell viability but combined deficiency of two genes leads to cell death. In cancer treatment, synthetic lethality is leveraged to exploit the dependency of cancer cells on a pathway that is essential for cell survival when a tumor suppressor is mutated. This approach enables pharmacological targeting of mutant tumor suppressors that are theoretically undruggable. Successful clinical introduction of BRCA-PARP synthetic lethality in cancer treatment led to additional discoveries of novel synthetic lethal partners of other tumor suppressors, including p53, PTEN, and RB1, using high-throughput screening. Recent work has highlighted aurora kinase A (AURKA) as a synthetic lethal partner of multiple tumor suppressors. AURKA is a serine/threonine kinase involved in a number of central biological processes, such as the G2/M transition, mitotic spindle assembly, and DNA replication. This review introduces synthetic lethal interactions between AURKA and its tumor suppressor partners and discusses the potential of AURKA inhibitors in precision cancer medicine.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Letai A. Functional precision cancer medicine-moving beyond pure genomics. Nat. Med. 2017;23:1028–1035. - PubMed
-
- Guarente L. Synthetic enhancement in gene interaction: a genetic tool come of age. Trends Genet. 1993;9:362–366. - PubMed
-
- Kaelin WG., Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer. 2005;5:689–698. - PubMed
-
- Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. - PubMed
-
- Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

