Hypothermia is not therapeutic in a neonatal piglet model of inflammation-sensitized hypoxia-ischemia
- PMID: 34050269
- PMCID: PMC8160560
- DOI: 10.1038/s41390-021-01584-6
Hypothermia is not therapeutic in a neonatal piglet model of inflammation-sensitized hypoxia-ischemia
Abstract
Background: Perinatal inflammation combined with hypoxia-ischemia (HI) exacerbates injury in the developing brain. Therapeutic hypothermia (HT) is standard care for neonatal encephalopathy; however, its benefit in inflammation-sensitized HI (IS-HI) is unknown.
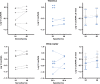
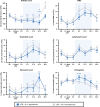
Methods: Twelve newborn piglets received a 2 µg/kg bolus and 1 µg/kg/h infusion over 52 h of Escherichia coli lipopolysaccharide (LPS). HI was induced 4 h after LPS bolus. After HI, piglets were randomized to HT (33.5 °C 1-25 h after HI, n = 6) or normothermia (NT, n = 6). Amplitude-integrated electroencephalogram (aEEG) was recorded and magnetic resonance spectroscopy (MRS) was acquired at 24 and 48 h. At 48 h, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive brain cell death, microglial activation/proliferation, astrogliosis, and cleaved caspase-3 (CC3) were quantified. Hematology and plasma cytokines were serially measured.
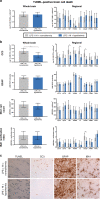
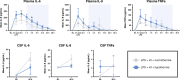
Results: Two HT piglets died. aEEG recovery, thalamic and white matter MRS lactate/N-acetylaspartate, and TUNEL-positive cell death were similar between groups. HT increased microglial activation in the caudate, but had no other effect on glial activation/proliferation. HT reduced CC3 overall. HT suppressed platelet count and attenuated leukocytosis. Cytokine profile was unchanged by HT.
Conclusions: We did not observe protection with HT in this piglet IS-HI model based on aEEG, MRS, and immunohistochemistry. Immunosuppressive effects of HT and countering neuroinflammation by LPS may contribute to the observed lack of HT efficacy. Other immunomodulatory strategies may be more effective in IS-HI.
Impact: Acute infection/inflammation is known to exacerbate perinatal brain injury and can worsen the outcomes in neonatal encephalopathy. Therapeutic HT is the current standard of care for all infants with NE, but the benefit in infants with coinfection/inflammation is unknown. In a piglet model of inflammation (LPS)-sensitized HI, we observed no evidence of neuroprotection with cooling for 24 h, based on our primary outcome measures: aEEG, MRS Lac/NAA, and histological brain cell death. Additional neuroprotective agents, with beneficial immunomodulatory effects, require exploration in IS-HI models.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

