Recipient sex and estradiol levels affect transplant outcomes in an age-specific fashion
- PMID: 34050595
- PMCID: PMC8924905
- DOI: 10.1111/ajt.16611
Recipient sex and estradiol levels affect transplant outcomes in an age-specific fashion
Abstract
Sex-specific influences have been shown for a variety of diseases. Whether donor or recipient sex and sex hormone levels impact alloimmune responses remains unclear. In unifactorial and multifactorial analyses of more than 400 000 SRTR listed kidney transplant patients, we found that younger female recipients had an inferior death-censored graft survival that was independent of donor sex. In contrast, graft survival was superior in older female recipients, suggesting the impact of recipient sex hormones over chromosomal sex mismatches. Those clinical changes were delineated in experimental skin and heart transplant models showing a prolongation of graft survival in ovariectomized young female recipients. In contrast, graft survival was comparable in ovariectomized and naïve old female recipients. Young ovariectomized mice showed reduced amounts and a compromised T cell proliferation. Deprivation of female hormones dampened the production of interferon (IFN)-γ and interleukin (IL)-17+ by CD4+ T cells while augmenting systemic counts of Tregs. Increasing estradiol concentrations in vitro promoted the switch of naïve CD4+ T cells into Th1 cells; high physiological estradiol concentrations dampening Th1 responses, promoted Tregs, and prolonged graft survival. Thus, clinical observations demonstrate age-specific graft survival patterns in female recipients. Estrogen levels, in turn, impact the fate of T cell subsets, providing relevant and novel information on age- and sex-specific alloimmunity.
Keywords: basic (laboratory) research/science; endocrinology/diabetology; gender; kidney failure/injury; kidney transplantation/nephrology; organ allocation; organ transplantation in general; rejection: T cell mediated (TCMR); reproductive biology.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
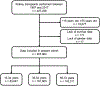


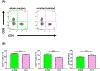




Comment in
-
Time for increased awareness of sex as a biological variable in transplantation.Am J Transplant. 2021 Oct;21(10):3215-3216. doi: 10.1111/ajt.16733. Epub 2021 Jul 12. Am J Transplant. 2021. PMID: 34174152 No abstract available.
-
Caution when using publicly available datasets.Am J Transplant. 2022 Feb;22(2):662-663. doi: 10.1111/ajt.16799. Epub 2021 Aug 23. Am J Transplant. 2022. PMID: 34390175 No abstract available.
References
-
- Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol 2014;35(3):347–369. - PubMed
-
- Regitz-Zagrosek V, Kararigas G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol Rev 2017;97(1):1–37. - PubMed
-
- McClelland EE, Smith JM. Gender specific differences in the immune response to infection. Arch Immunol Ther Exp (Warsz) 2011;59(3):203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

