NIAID ClinRegs-a Public Database of Country Clinical Research Regulatory and Ethics Requirements: Design and Utilization Analysis
- PMID: 34050748
- PMCID: PMC8492127
- DOI: 10.1093/cid/ciab505
NIAID ClinRegs-a Public Database of Country Clinical Research Regulatory and Ethics Requirements: Design and Utilization Analysis
Abstract
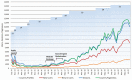
Regulatory compliance is challenging for multinational clinical trials. Conflicts between country requirements impedes research and slows the approval of medicines, leading the pharmaceutical industry to devote significant resources to this area. Many academic centers and nonprofits cannot support industry-level investment and are vulnerable to noncompliance. To address an insufficiency in public access to this information, the National Institute of Allergy and Infectious Diseases developed ClinRegs-a public access database of clinical research regulations. This report describes ClinRegs' features, maintenance, and usage. From September 2019 through August 2020, ClinRegs had 68 504 users, 60% from outside the United States, demonstrating the demand for accessible, reliable, country-specific regulatory information. Tools such as ClinRegs can help increase regulatory compliance and free up resources for research. We encourage our partner agencies and biomedical research industries to promote greater regulatory knowledge sharing and harmonization for the betterment of clinical research and improved public health.
Keywords: clinical research quality; clinical research regulations; clinical trial ethics; global clinical trials; public access.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures
References
-
- National Institutes of Health. Trends, charts, and maps. Available at: clinicaltrials.gov/ct2/resources/trends#RegisteredStudiesOverTime. Accessed 12 March 2020.
-
- Duley L, Antman K, Arena J, et al. . Specific barriers to the conduct of randomized trials. Clin Trials 2008; 5:40–8. - PubMed
-
- Califf RM. Clinical trials bureaucracy: unintended consequences of well-intentioned policy. Clin Trials 2006; 3:496–502. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

