The Role of Endoplasmic Reticulum Chaperones in Protein Folding and Quality Control
- PMID: 34050861
- PMCID: PMC9185992
- DOI: 10.1007/978-3-030-67696-4_3
The Role of Endoplasmic Reticulum Chaperones in Protein Folding and Quality Control
Abstract
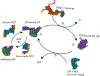
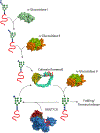
Molecular chaperones assist the folding of nascent chains in the cell. Chaperones also aid in quality control decisions as persistent chaperone binding can help to sort terminal misfolded proteins for degradation. There are two major molecular chaperone families in the endoplasmic reticulum (ER) that assist proteins in reaching their native structure and evaluating the fidelity of the maturation process. The ER Hsp70 chaperone, BiP, supports adenine nucleotide-regulated binding to non-native proteins that possess exposed hydrophobic regions. In contrast, the carbohydrate-dependent chaperone system involving the membrane protein calnexin and its soluble paralogue calreticulin recognize a specific glycoform of an exposed hydrophilic protein modification for which the composition is controlled by a series of glycosidases and transferases. Here, we compare and contrast the properties, mechanisms of action and functions of these different chaperones systems that work in parallel, as well as together, to assist a large variety of substrates that traverse the eukaryotic secretory pathway.
Keywords: Endoplasmic reticulum; Molecular chaperones; Quality control.
Conflict of interest statement
CONFLICT-OF-INTEREST DISCLOSURE STATEMENT
The authors declare that they have no conflicts of interest.
Figures
References
-
- Amin-Wetzel Niko, Saunders Reuben A, Kamphuis Maarten J, Rato Claudia, Preissler Steffen, Harding Heather P, and Ron David. 2017. “A J-Protein Co-Chaperone Recruits BiP to Monomerize IRE1 and Repress the Unfolded Protein Response.” Cell 171 (7): 1625–1637.e13. 10.1016/j.cell.2017.10.040. - DOI - PMC - PubMed
-
- Andréasson Claes, Rampelt Heike, Fiaux Jocelyne, Druffel-Augustin Silke, and Bukau Bernd. 2010. “The Endoplasmic Reticulum Grp170 Acts as a Nucleotide Exchange Factor of Hsp70 via a Mechanism Similar to That of the Cytosolic Hsp110.” The Journal of Biological Chemistry 285 (16): 12445–53. 10.1074/jbc.M109.096735. - DOI - PMC - PubMed
-
- Anfinsen Christian B. 1973. “Principles That Govern the Folding of Protein Chains.” Science 167 (3919): 886–87. https://doi.org/doi: 10.1126/science.181.4096.223. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

