Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study
- PMID: 34050932
- PMCID: PMC8239599
- DOI: 10.1002/cpt.2317
Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study
Abstract
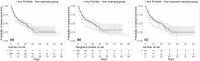
Several medications commonly used for a number of medical conditions share a property of functional inhibition of acid sphingomyelinase (ASM), or FIASMA. Preclinical and clinical evidence suggest that the ASM/ceramide system may be central to severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection. We examined the potential usefulness of FIASMA use among patients hospitalized for severe coronavirus disease 2019 (COVID-19) in an observational multicenter study conducted at Greater Paris University hospitals. Of 2,846 adult patients hospitalized for severe COVID-19, 277 (9.7%) were taking an FIASMA medication at the time of their hospital admission. The primary end point was a composite of intubation and/or death. We compared this end point between patients taking vs. not taking an FIASMA medication in time-to-event analyses adjusted for sociodemographic characteristics and medical comorbidities. The primary analysis was a Cox regression model with inverse probability weighting (IPW). Over a mean follow-up of 9.2 days (SD = 12.5), the primary end point occurred in 104 patients (37.5%) receiving an FIASMA medication, and 1,060 patients (41.4%) who did not. Despite being significantly and substantially associated with older age and greater medical severity, FIASMA medication use was significantly associated with reduced likelihood of intubation or death in both crude (hazard ratio (HR) = 0.71, 95% confidence interval (CI) = 0.58-0.87, P < 0.001) and primary IPW (HR = 0.58, 95%CI = 0.46-0.72, P < 0.001) analyses. This association remained significant in multiple sensitivity analyses and was not specific to one particular FIASMA class or medication. These results show the potential importance of the ASM/ceramide system in COVID-19 and support the continuation of FIASMA medications in these patients. Double-blind controlled randomized clinical trials of these medications for COVID-19 are needed.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
N.H., M.S.R., M.A., P.d.l.M., E.G., J.K., A.C., and F.L. are inventors on a patent application related to methods of treating COVID‐19, filled by Assistance Publique – Hopitaux de Paris in France. N.H. has received personal fees and nonfinancial support from Lundbeck, outside the submitted work. F.L. has received speaker and consulting fees from Janssen‐Cilag, Euthérapie‐Servier, and Lundbeck, outside the submitted work. E.L. has received grant support (non‐federal) from COVID Early Treatment Fund, Mercatus Center Emergent Ventures, the Skoll Foundation, the Taylor Family Institute for Innovative Psychiatric Research, the Center for Brain Research in Mood Disorders, the Patient‐Centered Outcomes Research Institute, Janssen, and the Barnes Jewish Foundation, and has received consulting fees from Janssen and Jazz Pharmaceuticals. A.R. has received grant or research support from the McDonnell Center for Systems Neuroscience, the McDonnell Center for Cellular and Molecular Neurobiology, and the Taylor Family Institute for Innovative Psychiatric Research. A.R. and E.L. are inventors on a patent application related to methods of treating COVID‐19, which was filed by Washington University in St. Louis. Other authors declare no conflict of interest related to this work.
Figures

Comment in
-
Compatibility of FIASMA Pharmacokinetics With Study End Points?Clin Pharmacol Ther. 2022 Feb;111(2):353. doi: 10.1002/cpt.2442. Epub 2021 Oct 31. Clin Pharmacol Ther. 2022. PMID: 34719015 No abstract available.
References
-
- Hoertel, N. et al. Facing the COVID‐19 epidemic in NYC: a stochastic agent‐based model of various intervention strategies. medRxiv 10.1101/2020.04.23.20076885. [e‐pub ahead of print]. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

