Efficacy comparison of three rapid antigen tests for SARS-CoV-2 and how viral load impact their performance
- PMID: 34050945
- PMCID: PMC8242364
- DOI: 10.1002/jmv.27108
Efficacy comparison of three rapid antigen tests for SARS-CoV-2 and how viral load impact their performance
Abstract
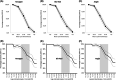
More and more rapid antigen tests for the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appear in the market with varying performance. The sensitivity of these tests heavily depends on the viral load, extrapolated by the threshold cycle (Ct). It is therefore essential to verify their performance before their inclusion in routine. The Coronavirus Ag Rapid Test Cassette Bio-Rad, the GSD NovaGen SARS-CoV-2 (COVID-19) Antigen Rapid Test, and the Aegle Coronavirus Ag Rapid Test Cassette were evaluated on 199 samples: 150 fresh samples from the routine and positive in quantitative reverse-transcription polymerase chain reaction (RT-qPCR), nine fresh samples negative in RT-qPCR, and 40 frozen samples, taken before the discovery of SARS-CoV-2 but positive for other respiratory viruses. Positive RT-qPCR samples were categorized according to their Ct: Ct < 20 (18.7%), ≥ 20-< 25 (27.3%), ≥ 25-< 30 (18.7%), ≥ 30-35 (17.3%), and > 35 (18.0%). Sensitivities (95% confidence interval) for Ct below 25 were 95.7% (92.4-98.9), 97.1% (94.4-99.8), and 97.1% (94.4-99.8) for GSD NovaGen, Bio-Rad, and Aegle, respectively but drastically dropped when Ct exceeded 27. Among samples with previously diagnosed viruses, seven false-positive results were found with GSD NovaGen only (specificity 85.7%). Equivalent, high sensitivities were observed with the highest viral load samples. The GSD NovaGen assay showed less specificity. Although the three kits tested in this study are inadequate for routine testing in a high throughput laboratory, they can help to quickly identify the most infectious patients and screen their close contacts in an environment where molecular tests are not readily available.
Keywords: COVID-19; SARS-CoV-2; point-of-care testing; rapid antigen test.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors have no relevant competing interest to disclose in relation to this study.
Figures
References
-
- Diao B, Wen K, Chen J, et al. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein [published online ahead of print March 13, 2020]. Epidemiology. 2020. 10.1101/2020.03.07.20032524 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous