Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial
- PMID: 34051124
- PMCID: PMC8361994
- DOI: 10.1002/ejhf.2249
Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial
Abstract
Aims: Sodium-glucose co-transporter 2 (SGLT2) inhibitors, originally developed as glucose-lowering agents, have been shown to reduce heart failure hospitalizations in patients with type 2 diabetes without established heart failure, and in patients with heart failure with and without diabetes. Their role in patients with heart failure with preserved and mildly reduced ejection fraction remains unknown.
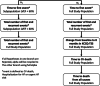
Methods: Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER) is an international, multicentre, parallel group, event-driven, randomized, double-blind trial in patients with chronic heart failure and left ventricular ejection fraction (LVEF) >40%, comparing the effect of dapagliflozin 10 mg once daily, vs. placebo, in addition to standard of care. Patients with or without diabetes, with signs and symptoms of heart failure, a LVEF >40%, elevation in natriuretic peptides and evidence of structural heart disease are eligible. The primary endpoint is time-to-first cardiovascular death or worsening heart failure event (heart failure hospitalization or urgent heart failure visit), and will be assessed in dual primary analyses - the full population and in those with LVEF <60%. The study is event-driven and will target 1117 primary events. A total of 6263 patients have been randomized.
Conclusions: DELIVER will determine the efficacy and safety of the SGLT2 inhibitor dapagliflozin, added to conventional therapy, in patients with heart failure and preserved and mildly reduced ejection fraction.
Keywords: Heart failure with preserved ejection fraction; Sodium-glucose co-transporter 2 inhibitors.
© 2021 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures

References
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. - PubMed
-
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. - PubMed
-
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

