Dysbiosis exacerbates colitis by promoting ubiquitination and accumulation of the innate immune adaptor STING in myeloid cells
- PMID: 34051146
- PMCID: PMC8237382
- DOI: 10.1016/j.immuni.2021.05.008
Dysbiosis exacerbates colitis by promoting ubiquitination and accumulation of the innate immune adaptor STING in myeloid cells
Abstract
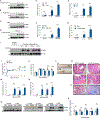
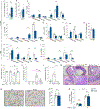
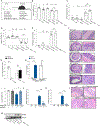
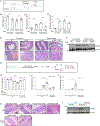
Alterations in the cGAS-STING DNA-sensing pathway affect intestinal homeostasis. We sought to delineate the functional role of STING in intestinal inflammation. Increased STING expression was a feature of intestinal inflammation in mice with colitis and in humans afflicted with inflammatory bowel disease. Mice bearing an allele rendering STING constitutively active exhibited spontaneous colitis and dysbiosis, as well as progressive chronic intestinal inflammation and fibrosis. Bone marrow chimera experiments revealed STING accumulation in intestinal macrophages and monocytes as the initial driver of inflammation. Depletion of Gram-negative bacteria prevented STING accumulation in these cells and alleviated intestinal inflammation. STING accumulation occurred at the protein rather than transcript level, suggesting post-translational stabilization. We found that STING was ubiquitinated in myeloid cells, and this K63-linked ubiquitination could be elicited by bacterial products, including cyclic di-GMP. Our findings suggest a positive feedback loop wherein dysbiosis foments the accumulation of STING in intestinal myeloid cells, driving intestinal inflammation.
Keywords: SAVI; STING; colitis; colon; commensal; dysbiosis; intestinal inflammation; microbiome; myeloid cells; ubiquitination.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no competing interests to declare.
Figures



References
-
- ADEN K, TRAN F, ITO G, SHEIBANI-TEZERJI R, LIPINSKI S, KUIPER JW, TSCHURTSCHENTHALER M, SAVELJEVA S, BHATTACHARYYA J, HASLER R, BARTSCH K, LUZIUS A, JENTZSCH M, FALK-PAULSEN M, STENGEL ST, WELZ L, SCHWARZER R, RABE B, BARCHET W, KRAUTWALD S, HARTMANN G, PASPARAKIS M, BLUMBERG RS, SCHREIBER S, KASER A & ROSENSTIEL P 2018. ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med, 215, 2868–2886. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

