Does the number of doses matter? A qualitative study of HPV vaccination acceptability nested in a dose reduction trial in Tanzania
- PMID: 34051389
- PMCID: PMC8233223
- DOI: 10.1016/j.tvr.2021.200217
Does the number of doses matter? A qualitative study of HPV vaccination acceptability nested in a dose reduction trial in Tanzania
Abstract
Background: The multi-dose regimen is a known barrier to successful human papillomavirus (HPV) vaccination. Emerging evidence suggests that one vaccine dose could protect against HPV. While there are clear advantages to a single dose schedule, beliefs about vaccine dosage in low and middle income countries (LMICs) are poorly understood. We investigated acceptability of dose-reduction among girls, and parents/guardians of girls, randomised to receive one, two or three doses in an HPV vaccine dose-reduction and immunobridging study (DoRIS trial) in Tanzania.
Methods: Semi-structured interviews with girls (n = 19), and parents/guardians of girls (n = 18), enrolled in the study and completing their vaccine course.
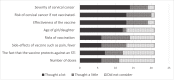
Results: Most participants said they entrusted decisions about the number of HPV vaccine doses to experts. Random allocation to the different dose groups did not feature highly in the decision to participate in the trial. Given a hypothetical choice, girls generally said they would prefer fewer doses in order to avoid the pain of injections. Parental views were mixed, with most wanting whichever dose was most efficacious. Nonetheless, a few parents equated a higher number of doses with greater protection.
Conclusion: Vaccine trials and programmes will need to employ careful messaging to explain that one dose offers sufficient protection against HPV should emerging evidence from ongoing dose-reduction clinical trials support this.
Keywords: Acceptability; Dose reduction; HPV vaccination; Qualitative; Randomisation; Trial participation.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Comparing one dose of HPV vaccine in girls aged 9-14 years in Tanzania (DoRIS) with one dose of HPV vaccine in historical cohorts: an immunobridging analysis of a randomised controlled trial.Lancet Glob Health. 2022 Oct;10(10):e1485-e1493. doi: 10.1016/S2214-109X(22)00306-0. Lancet Glob Health. 2022. PMID: 36113532 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of one-dose human papillomavirus vaccine compared with two or three doses in Tanzanian girls (DoRIS): an open-label, randomised, non-inferiority trial.Lancet Glob Health. 2022 Oct;10(10):e1473-e1484. doi: 10.1016/S2214-109X(22)00309-6. Lancet Glob Health. 2022. PMID: 36113531 Free PMC article. Clinical Trial.
-
A dose-reduction HPV vaccine immunobridging trial of two HPV vaccines among adolescent girls in Tanzania (the DoRIS trial) - Study protocol for a randomised controlled trial.Contemp Clin Trials. 2021 Feb;101:106266. doi: 10.1016/j.cct.2021.106266. Epub 2021 Jan 6. Contemp Clin Trials. 2021. PMID: 33421649 Free PMC article.
-
Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males.Cochrane Database Syst Rev. 2019 Nov 22;2019(11):CD013479. doi: 10.1002/14651858.CD013479. Cochrane Database Syst Rev. 2019. PMID: 31755549 Free PMC article.
-
National introduction of human papillomavirus (HPV) vaccine in Tanzania: Programmatic decision-making and implementation.Vaccine. 2022 Mar 31;40 Suppl 1(Suppl 1):A2-A9. doi: 10.1016/j.vaccine.2021.04.025. Epub 2021 May 4. Vaccine. 2022. PMID: 33962839 Free PMC article. Review.
Cited by
-
Meta-analysis of the correlation between vaginal microenvironment and HPV infection.Am J Transl Res. 2023 Feb 15;15(2):630-640. eCollection 2023. Am J Transl Res. 2023. PMID: 36915746 Free PMC article. Review.
-
Impact of social determinants and medical mistrust on parent-child HPV vaccination in economically disadvantaged communities: implications for cancer prevention.Front Oncol. 2025 Jan 23;14:1422839. doi: 10.3389/fonc.2024.1422839. eCollection 2024. Front Oncol. 2025. PMID: 39917363 Free PMC article.
-
Factors that influence caregivers' and adolescents' views and practices regarding human papillomavirus (HPV) vaccination for adolescents: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2025 Apr 15;4(4):CD013430. doi: 10.1002/14651858.CD013430.pub2. Cochrane Database Syst Rev. 2025. PMID: 40232221 Free PMC article.
-
Ethical issues in vaccine trial participation by adolescents: qualitative insights on family decision making from a human papillomavirus vaccine trial in Tanzania.BMC Med Ethics. 2024 Nov 20;25(1):134. doi: 10.1186/s12910-024-01122-z. BMC Med Ethics. 2024. PMID: 39563292 Free PMC article.
References
-
- SAGE Meeting of the strategic advisory group of experts on immunization, october 2018 - conclusions and recommendations. Wkly. Epidemiol. Rec. 2018;49:661–680.
-
- PATH . 2020. Global HPV Vaccine Introduction Overview.https://path.azureedge.net/media/documents/Global_HPV_Vaccine_Intro_Over... (last accessed June 14th, 2020)
-
- Bruni L., Diaz M., Barrionuevo-Rosas L., Herrero R., Bray F., Bosch F.X., de Sanjosé S., Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. The Lancet Global Health. 2016;4(7):e453–e463. - PubMed
-
- Kessels S.J., Marshall H.S., Watson M., Braunack-Mayer A.J., Reuzel R., Tooher R.L. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30(24):3546–3556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical