Characterizing initiation, use, and discontinuation of extended-release buprenorphine in a nationally representative United States commercially insured cohort
- PMID: 34051547
- PMCID: PMC8488795
- DOI: 10.1016/j.drugalcdep.2021.108764
Characterizing initiation, use, and discontinuation of extended-release buprenorphine in a nationally representative United States commercially insured cohort
Abstract
Background and aims: While the United States is in the midst of an overdose epidemic, effective treatments are underutilized and commonly discontinued. Innovations in medication delivery, including an extended-release formulations, have the potential to improve treatment access and reduce discontinuation. We sought to assess extended-release buprenorphine discontinuation among individuals with opioid use disorder (OUD) in a real-world, nationally representative cohort.
Setting: United States PARTICIPANTS: Commercially insured individuals initiating one of four FDA-approved medications for opioid use disorder (MOUD) in 2018: extended-release buprenorphine, extended-release naltrexone, mucosal buprenorphine (mono- or co-formulated with naloxone), or methadone.
Measurements: Our primary outcome was medication discontinuation, defined as a gap of more than 14 days between the end of one prescription or administration and the subsequent dose.
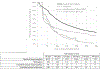
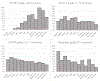
Findings: We identified 14,358 individuals initiating MOUD in 2018, including 204 (1%) extended-release buprenorphine, 1,173 (8%) extended-release naltrexone, 12,171 (85%) mucosal buprenorphine, and 810 (6%) methadone initiations. Three months after initiation, 50% (95% confidence interval [CI] 40%-60%) of extended-release buprenorphine, 64% (95% CI 61%-69%) of extended-release naltrexone, 34% (95% CI 33%-35%) of mucosal buprenorphine, and 58% (95% CI 54%-62%) of methadone initiators had discontinued treatment.
Conclusions: Across all treatment groups, medication discontinuation was high, and in this sample of early adopters with limited follow-up time, we found no evidence that extended-release buprenorphine offered a retention advantage compared to other MOUD in real-world settings. Retention continues to represent a major obstacle to treatment effectiveness, and interventions are needed to address this challenge even as new MOUD formulations become available.
Keywords: Extended-release buprenorphine; Medication for opioid Use disorder; Opioid use disorder; Retention.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures

References
-
- Gladden RM, Martinez P, Seth P. Fentanyl Law Enforcement Submissions and Increases in Synthetic Opioid-Involved Overdose Deaths - 27 States, 2013–2014. MMWR Morb Mortal Wkly Rep 2016;65:837–43. - PubMed
-
- Davenport S, Matthews K. Opioid use disorder in the United States: Diagnosed prevalence by payer, age, sex, and state Washington, DC: Milliman; 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

