Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection
- PMID: 34051613
- PMCID: PMC8133385
- DOI: 10.1016/j.jaut.2021.102662
Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection
Abstract
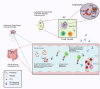
Herein, we consider venous immunothrombotic mechanisms in SARS-CoV-2 infection and anti-SARS-CoV-2 DNA vaccination. Primary SARS-CoV-2 infection with systemic viral RNA release (RNAaemia) contributes to innate immune coagulation cascade activation, with both pulmonary and systemic immunothrombosis - including venous territory strokes. However, anti-SARS-CoV-2 adenoviral-vectored-DNA vaccines -initially shown for the ChAdOx1 vaccine-may rarely exhibit autoimmunity with autoantibodies to Platelet Factor-4 (PF4) that is termed Vaccine-Induced Thrombotic Thrombocytopenia (VITT), an entity pathophysiologically similar to Heparin-Induced Thrombocytopenia (HIT). The PF4 autoantigen is a polyanion molecule capable of independent interactions with negatively charged bacterial cellular wall, heparin and DNA molecules, thus linking intravascular innate immunity to both bacterial cell walls and pathogen-derived DNA. Crucially, negatively charged extracellular DNA is a powerful adjuvant that can break tolerance to positively charged nuclear histone proteins in many experimental autoimmunity settings, including SLE and scleroderma. Analogous to DNA-histone interactons, positively charged PF4-DNA complexes stimulate strong interferon responses via Toll-Like Receptor (TLR) 9 engagement. A chain of events following intramuscular adenoviral-vectored-DNA vaccine inoculation including microvascular damage; microbleeding and platelet activation with PF4 release, adenovirus cargo dispersement with DNA-PF4 engagement may rarely break immune tolerance, leading to rare PF4-directed autoimmunity. The VITT cavernous sinus cerebral and intestinal venous territory immunothrombosis proclivity may pertain to venous drainage of shared microbiotal-rich areas of the nose and in intestines that initiates local endovascular venous immunity by PF4/microbiotal engagement with PF4 autoantibody driven immunothrombosis reminiscent of HIT. According to the proposed model, any adenovirus-vectored-DNA vaccine could drive autoimmune VITT in susceptible individuals and alternative mechanism based on molecular mimicry, vaccine protein contaminants, adenovirus vector proteins, EDTA buffers or immunity against the viral spike protein are secondary factors. Hence, electrochemical DNA-PF4 interactions and PF4-heparin interactions, but at different locations, represent the common denominator in HIT and VITT related autoimmune-mediated thrombosis.
Keywords: COVID-19 pneumonia related thrombosis; Heparin induced thrombocytopenia (HIT) DNA-PF4 interactions. VITT model; Vaccine induced thrombotic thrombocytopenia (VITT).
Published by Elsevier Ltd.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

