Biomimetic tubular scaffold with heparin conjugation for rapid degradation in in situ regeneration of a small diameter neoartery
- PMID: 34051629
- PMCID: PMC8390125
- DOI: 10.1016/j.biomaterials.2021.120874
Biomimetic tubular scaffold with heparin conjugation for rapid degradation in in situ regeneration of a small diameter neoartery
Abstract
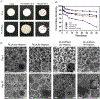
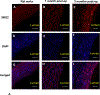
To address the clinical need for readily available small diameter vascular grafts, biomimetic tubular scaffolds were developed for rapid in situ blood vessel regeneration. The tubular scaffolds were designed to have an inner layer that is porous, interconnected, and with a nanofibrous architecture, which provided an excellent microenvironment for host cell invasion and proliferation. Through the synthesis of poly(spirolactic-co-lactic acid) (PSLA), a highly functional polymer with a norbornene substituting a methyl group in poly(l-lactic acid) (PLLA), we were able to covalently attach biomolecules onto the polymer backbone via thiol-ene click chemistry to impart desirable functionalities to the tubular scaffolds. Specifically, heparin was conjugated on the scaffolds in order to prevent thrombosis when implanted in situ. By controlling the amount of covalently attached heparin we were able to modulate the physical properties of the tubular scaffold, resulting in tunable wettability and degradation rate while retaining the porous and nanofibrous morphology. The scaffolds were successfully tested as rat abdominal aortic replacements. Patency and viability were confirmed through dynamic ultrasound and histological analysis of the regenerated tissue. The harvested tissue showed excellent vascular cellular infiltration, proliferation, and migration with laminar cellular arrangement. Furthermore, we achieved both complete reendothelialization of the vessel lumen and native-like media extracellular matrix. No signs of aneurysm or hyperplasia were observed after 3 months of vessel replacement. Taken together, we have developed an effective vascular graft able to generate small diameter blood vessels that can function in a rat model.
Keywords: Heparin conjugation; Small diameter vascular graft; Tubular scaffold; Vascular smooth muscle cells; Vascular tissue engineering.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
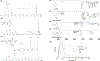






References
-
- Matheny M; McPheeters ML; Glasser A; Mercaldo N; Weaver RB; Jerome RN; Walden R; McKoy JN; Pritchett J; Tsai C, In Systematic Review of Cardiovascular Disease Risk Assessment Tools, Rockville (MD), 2011. - PubMed
-
- Kaplan GA; Keil JE, Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993, 88 (4 Pt 1), 1973–98. - PubMed
-
- In Health, United States, 2010: With Special Feature on Death and Dying, Hyattsville (MD), 2011. - PubMed
-
- Ercolani E; Del Gaudio C; Bianco A, Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach. J Tissue Eng Regen Med 2015, 9 (8), 861–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

