Oleaginous yeasts respond differently to carbon sources present in lignocellulose hydrolysate
- PMID: 34051838
- PMCID: PMC8164748
- DOI: 10.1186/s13068-021-01974-2
Oleaginous yeasts respond differently to carbon sources present in lignocellulose hydrolysate
Abstract
Background: Microbial oils, generated from lignocellulosic material, have great potential as renewable and sustainable alternatives to fossil-based fuels and chemicals. By unravelling the diversity of lipid accumulation physiology in different oleaginous yeasts grown on the various carbon sources present in lignocellulose hydrolysate (LH), new targets for optimisation of lipid accumulation can be identified. Monitoring lipid formation over time is essential for understanding lipid accumulation physiology. This study investigated lipid accumulation in a variety of oleaginous ascomycetous and basidiomycetous strains grown in glucose and xylose and followed lipid formation kinetics of selected strains in wheat straw hydrolysate (WSH).
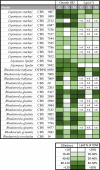
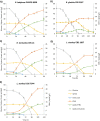
Results: Twenty-nine oleaginous yeast strains were tested for their ability to utilise glucose and xylose, the main sugars present in WSH. Evaluation of sugar consumption and lipid accumulation revealed marked differences in xylose utilisation capacity between the yeast strains, even between those belonging to the same species. Five different promising strains, belonging to the species Lipomyces starkeyi, Rhodotorula glutinis, Rhodotorula babjevae and Rhodotorula toruloides, were grown on undiluted wheat straw hydrolysate and lipid accumulation was followed over time, using Fourier transform-infrared (FTIR) spectroscopy. All five strains were able to grow on undiluted WSH and to accumulate lipids, but to different extents and with different productivities. R. babjevae DVBPG 8058 was the best-performing strain, accumulating 64.8% of cell dry weight (CDW) as lipids. It reached a culture density of 28 g/L CDW in batch cultivation, resulting in a lipid content of 18.1 g/L and yield of 0.24 g lipids per g carbon source. This strain formed lipids from the major carbon sources in hydrolysate, glucose, acetate and xylose. R. glutinis CBS 2367 also consumed these carbon sources, but when assimilating xylose it consumed intracellular lipids simultaneously. Rhodotorula strains contained a higher proportion of polyunsaturated fatty acids than the two tested Lipomyces starkeyi strains.
Conclusions: There is considerable metabolic diversity among oleaginous yeasts, even between closely related species and strains, especially when converting xylose to biomass and lipids. Monitoring the kinetics of lipid accumulation and identifying the molecular basis of this diversity are keys to selecting suitable strains for high lipid production from lignocellulose.
Keywords: Ascomycetes; Basidiomycetes; Biofuels; FTIR; Lignocellulose; Lipids; Oleaginous yeasts; Xylose.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- BP. BP Statistical Review of World Energy 2019, No. 68. British Petroleum. https://www.bp.com/en/global/corporate/energy-economics/statistical-revi.... 2019(69). Accessed 26 April 2021.
-
- Knothe G. “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels. 2008;22(2):1358–1364. doi: 10.1021/ef700639e. - DOI
-
- Atabani AE, Silitonga AS, Badruddin IA, Mahlia T, Masjuki H, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev. 2012;16(4):2070–2093. doi: 10.1016/j.rser.2012.01.003. - DOI
-
- Anuar MR, Abdullah AZ. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: a critical review. Renew Sustain Energy Rev. 2016;58:208–223. doi: 10.1016/j.rser.2015.12.296. - DOI
-
- Passoth V. Molecular mechanisms in yeast carbon metabolism. Berlin: Springer; 2014. Molecular mechanisms in yeast carbon metabolism: bioethanol and other biofuels; pp. 217–259.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

