Current understanding of epigenetics mechanism as a novel target in reducing cancer stem cells resistance
- PMID: 34051847
- PMCID: PMC8164819
- DOI: 10.1186/s13148-021-01107-4
Current understanding of epigenetics mechanism as a novel target in reducing cancer stem cells resistance
Abstract
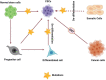
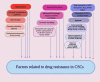
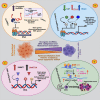
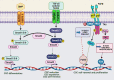
At present, after extensive studies in the field of cancer, cancer stem cells (CSCs) have been proposed as a major factor in tumor initiation, progression, metastasis, and recurrence. CSCs are a subpopulation of bulk tumors, with stem cell-like properties and tumorigenic capabilities, having the abilities of self-renewal and differentiation, thereby being able to generate heterogeneous lineages of cancer cells and lead to resistance toward anti-tumor treatments. Highly resistant to conventional chemo- and radiotherapy, CSCs have heterogeneity and can migrate to different organs and metastasize. Recent studies have demonstrated that the population of CSCs and the progression of cancer are increased by the deregulation of different epigenetic pathways having effects on gene expression patterns and key pathways connected with cell proliferation and survival. Further, epigenetic modifications (DNA methylation, histone modifications, and RNA methylations) have been revealed to be key drivers in the formation and maintenance of CSCs. Hence, identifying CSCs and targeting epigenetic pathways therein can offer new insights into the treatment of cancer. In the present review, recent studies are addressed in terms of the characteristics of CSCs, the resistance thereof, and the factors influencing the development thereof, with an emphasis on different types of epigenetic changes in genes and main signaling pathways involved therein. Finally, targeted therapy for CSCs by epigenetic drugs is referred to, which is a new approach in overcoming resistance and recurrence of cancer.
Keywords: Cancer stem cell (CSC); Drug resistance; Epi-drugs; Epigenetic modifications; Signaling pathway.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Seoane J. Division hierarchy leads to cell heterogeneity. Nature. 2017;549:164–166. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

