Same-day SARS-CoV-2 antigen test screening in an indoor mass-gathering live music event: a randomised controlled trial
- PMID: 34051886
- PMCID: PMC8457773
- DOI: 10.1016/S1473-3099(21)00268-1
Same-day SARS-CoV-2 antigen test screening in an indoor mass-gathering live music event: a randomised controlled trial
Abstract
Background: The banning of mass-gathering indoor events to prevent SARS-CoV-2 spread has had an important effect on local economies. Despite growing evidence on the suitability of antigen-detecting rapid diagnostic tests (Ag-RDT) for mass screening at the event entry, this strategy has not been assessed under controlled conditions. We aimed to assess the effectiveness of a prevention strategy during a live indoor concert.
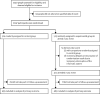
Methods: We designed a randomised controlled open-label trial to assess the effectiveness of a comprehensive preventive intervention for a mass-gathering indoor event (a live concert) based on systematic same-day screening of attendees with Ag-RDTs, use of facial masks, and adequate air ventilation. The event took place in the Sala Apolo, Barcelona, Spain. Adults aged 18-59 years with a negative result in an Ag-RDT from a nasopharyngeal swab collected immediately before entering the event were randomised 1:1 (block randomisation stratified by age and gender) to either attend the indoor event for 5 hours or go home. Nasopharyngeal specimens used for Ag-RDT screening were analysed by real-time reverse-transcriptase PCR (RT-PCR) and cell culture (Vero E6 cells). 8 days after the event, a nasopharyngeal swab was collected and analysed by Ag-RDT, RT-PCR, and a transcription-mediated amplification test (TMA). The primary outcome was the difference in incidence of RT-PCR-confirmed SARS-CoV-2 infection at 8 days between the control and the intervention groups, assessed in all participants who were randomly assigned, attended the event, and had a valid result for the SARS-CoV-2 test done at follow-up. The trial is registered at ClinicalTrials.gov, NCT04668625.
Findings: Participant enrollment took place during the morning of the day of the concert, Dec 12, 2020. Of the 1140 people who responded to the call and were deemed eligible, 1047 were randomly assigned to either enter the music event (experimental group) or continue with normal life (control group). Of the 523 randomly assigned to the experimental group, 465 were included in the analysis of the primary outcome (51 did not enter the event and eight did not take part in the follow-up assessment), and of the 524 randomly assigned to the control group, 495 were included in the final analysis (29 did not take part in the follow-up). At baseline, 15 (3%) of 495 individuals in the control group and 13 (3%) of 465 in the experimental group tested positive on TMA despite a negative Ag-RDT result. The RT-PCR test was positive in one case in each group and cell viral culture was negative in all cases. 8 days after the event, two (<1%) individuals in the control arm had a positive Ag-RDT and PCR result, whereas no Ag-RDT nor RT-PCR positive results were found in the intervention arm. The Bayesian estimate for the incidence between the experimental and control groups was -0·15% (95% CI -0·72 to 0·44).
Interpretation: Our study provides preliminary evidence on the safety of indoor mass-gathering events during a COVID-19 outbreak under a comprehensive preventive intervention. The data could help restart cultural activities halted during COVID-19, which might have important sociocultural and economic implications.
Funding: Primavera Sound Group and the #YoMeCorono Initiative.
Translation: For the Spanish translation of the abstract see Supplementary Materials section.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests PS is an employee and stockholder of Primavera Sound, sponsor of the study. All other authors declare no competing interests.
Comment in
-
Innovations in COVID-19 testing: the road from pandemic response to control.Lancet Infect Dis. 2021 Oct;21(10):1334-1335. doi: 10.1016/S1473-3099(21)00291-7. Epub 2021 May 27. Lancet Infect Dis. 2021. PMID: 34051885 Free PMC article. No abstract available.
-
Practical measures for SARS-CoV-2 infection prevention.Lancet Infect Dis. 2022 Jan;22(1):20-21. doi: 10.1016/S1473-3099(21)00769-6. Lancet Infect Dis. 2022. PMID: 34953547 Free PMC article. No abstract available.
-
Practical measures for SARS-CoV-2 infection prevention - Authors' reply.Lancet Infect Dis. 2022 Jan;22(1):21. doi: 10.1016/S1473-3099(21)00766-0. Lancet Infect Dis. 2022. PMID: 34953549 Free PMC article. No abstract available.
References
-
- Adam DC, Wu P, Wong JY, et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714–1719. - PubMed
-
- Centers for Disease Control Events and gatherings: readiness and planning tool. 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/large-events/conside...
-
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous