Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance
- PMID: 34052208
- PMCID: PMC8212140
- DOI: 10.1016/j.redox.2021.101953
Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance
Abstract
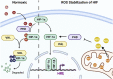
Controlling reactive oxygen species (ROS) at sustainable levels can drive multiple facets of tumor biology, including within the cancer stem cell (CSC) population. Tight regulation of ROS is one key component in CSCs that drives disease recurrence, cell signaling, and therapeutic resistance. While ROS are well-appreciated to need oxygen and are a product of oxidative phosphorylation, there are also important roles for ROS under hypoxia. As hypoxia promotes and sustains major stemness pathways, further consideration of ROS impacts on CSCs in the tumor microenvironment is important. Furthermore, glycolytic shifts that occur in cancer and may be promoted by hypoxia are associated with multiple mechanisms to mitigate oxidative stress. This altered metabolism provides survival advantages that sustain malignant features, such as proliferation and self-renewal, while producing the necessary antioxidants that reduce damage from oxidative stress. Finally, disease recurrence is believed to be attributed to therapy resistant CSCs which can be quiescent and have changes in redox status. Effective DNA damage response pathways and/or a slow-cycling state can protect CSCs from the genomic catastrophe induced by irradiation and genotoxic agents. This review will explore the delicate, yet complex, relationship between ROS and its pleiotropic role in modulating the CSC.
Keywords: Cancer stem cell; Metabolism; Reactive oxygen species; Tumor initiating cell.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
We have no conflicts of interest to declare.
Figures



Similar articles
-
Prx2 links ROS homeostasis to stemness of cancer stem cells.Free Radic Biol Med. 2019 Apr;134:260-267. doi: 10.1016/j.freeradbiomed.2019.01.001. Epub 2019 Jan 4. Free Radic Biol Med. 2019. PMID: 30611866
-
Targeting CSCs in tumor microenvironment: the potential role of ROS-associated miRNAs in tumor aggressiveness.Curr Stem Cell Res Ther. 2014 Jan;9(1):22-35. doi: 10.2174/1574888x113089990053. Curr Stem Cell Res Ther. 2014. PMID: 23957937 Free PMC article. Review.
-
Strategies to Tackle Radiation Resistance by Penetrating Cancer Stem Cell Line of Scrimmage.Recent Pat Anticancer Drug Discov. 2018;13(1):18-39. doi: 10.2174/1574892812666171003150410. Recent Pat Anticancer Drug Discov. 2018. PMID: 28971778 Review.
-
Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells.Oxid Med Cell Longev. 2015;2015:425708. doi: 10.1155/2015/425708. Epub 2015 Apr 29. Oxid Med Cell Longev. 2015. PMID: 26064420 Free PMC article. Review.
-
Targeting redox regulation and autophagy systems in cancer stem cells.Clin Exp Med. 2023 Sep;23(5):1405-1423. doi: 10.1007/s10238-022-00955-5. Epub 2022 Dec 6. Clin Exp Med. 2023. PMID: 36473988 Review.
Cited by
-
Emerging Role of NRF2 Signaling in Cancer Stem Cell Phenotype.Mol Cells. 2023 Mar 31;46(3):153-164. doi: 10.14348/molcells.2023.2196. Epub 2023 Mar 27. Mol Cells. 2023. PMID: 36994474 Free PMC article. Review.
-
Molecular Mechanisms of Dietary Compounds in Cancer Stem Cells from Solid Tumors: Insights into Colorectal, Breast, and Prostate Cancer.Int J Mol Sci. 2025 Jan 13;26(2):631. doi: 10.3390/ijms26020631. Int J Mol Sci. 2025. PMID: 39859345 Free PMC article. Review.
-
Redox-Regulation in Cancer Stem Cells.Biomedicines. 2022 Sep 27;10(10):2413. doi: 10.3390/biomedicines10102413. Biomedicines. 2022. PMID: 36289675 Free PMC article. Review.
-
Identification of Quiescent Cells in a Zebrafish T-Cell Acute Lymphoblastic Leukemia Model Using Cell Proliferation Staining.J Vis Exp. 2024 Jul 19;(209):10.3791/67059. doi: 10.3791/67059. J Vis Exp. 2024. PMID: 39141548 Free PMC article.
-
Strategies in the development of pro-oxidant therapy for oral squamous cell carcinoma: A scoping review.J Taibah Univ Med Sci. 2025 Jun 27;20(3):417-428. doi: 10.1016/j.jtumed.2025.06.002. eCollection 2025 Jun. J Taibah Univ Med Sci. 2025. PMID: 40678631 Free PMC article. Review.
References
-
- Weissman I.L. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. - PubMed
-
- Till J.E., McCulloch E.A. Hemopoietic stem cell differentiation. Biochim. Biophys. Acta. 1980;605(4):431–459. - PubMed
-
- Morrison S.J., Shah N.M., Anderson D.J. Regulatory mechanisms in stem cell biology. Cell. 1997;88(3):287–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

