Responses of neurons in rostral ventromedial medulla to nociceptive stimulation of craniofacial region and tail in rats
- PMID: 34052258
- PMCID: PMC8349861
- DOI: 10.1016/j.brainres.2021.147539
Responses of neurons in rostral ventromedial medulla to nociceptive stimulation of craniofacial region and tail in rats
Abstract
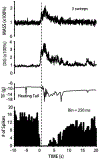
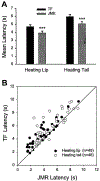
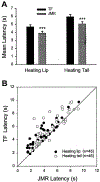
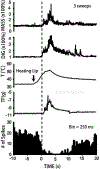
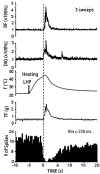
The rostral ventromedial medulla (RVM) plays a key role in the endogenous modulation of nociceptive transmission in the central nervous system (CNS). The primary aim of this study was to examine whether the activities of RVM neurons were related to craniofacial nociceptive behaviour (jaw-motor response, JMR) as well as the tail-flick response (TF). The activities of RVM neurons and TF and JMR evoked by noxious heating of the tail or perioral skin were recorded simultaneously in lightly anaesthetized rats. Tail or perioral heating evoked the TF and JMR, and the latency of the JMR was significantly shorter (P < 0.001) than that of the TF. Of 89 neurons recorded in RVM, 40 were classified as ON-cells, 27 as OFF-cells, and 22 as NEUTRAL-cells based on their responsiveness to heating of the tail. Heating at either site caused an increase in ON-cell and decrease in OFF-cell activity before the occurrence of the TF and JMR, but did not alter the activity of NEUTRAL cells. Likewise, noxious stimulation of the temporomandibular joint had similar effects on RVM neurons. These findings reveal that the JMR is a measure of the excitability of trigeminal and spinal nociceptive circuits in the CNS, and that the JMR as well as TF can be used for studying processes related to descending modulation of pain. The findings also support the view that RVM ON- and OFF-cells play an important role in the elaboration of diverse nociceptive behaviours evoked by noxious stimulation of widely separated regions of the body.
Keywords: Craniofacial; Descending modulation; Neurons; Pain; Rostral ventromedial medulla.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors has a conflict of interest to declare.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

