Effect of statin treatment in obese selenium-supplemented mice lacking selenocysteine lyase
- PMID: 34052303
- PMCID: PMC8263501
- DOI: 10.1016/j.mce.2021.111335
Effect of statin treatment in obese selenium-supplemented mice lacking selenocysteine lyase
Abstract
People with obesity are often dyslipidemic and prescribed statins to prevent cardiovascular events. A common side effect of statin use is myopathy. This could potentially be caused by the reduction of selenoproteins that curb oxidative stress, in turn, affecting creatine metabolism. We determined if statins regulate hepatic and muscular selenoprotein expression, oxidative stress and creatine metabolism. Mice lacking selenocysteine lyase (Scly KO), a selenium-provider enzyme for selenoprotein synthesis, were fed a high-fat, Se-supplemented diet and treated with simvastatin. Statin improved creatine metabolism in females and oxidative responses in both sexes. Male Scly KO mice were heavier than females after statin treatment. Hepatic selenoproteins were unaffected by statin and genotype in females. Statin upregulated muscular Gpx1 in females but not males, while Scly loss downregulated muscular Gpx1 in males and Selenon in females. Osgin1 was reduced in statin-treated Scly KO males after AmpliSeq analysis. These results refine our understanding of the sex-dependent role of selenium in statin responses.
Keywords: Obesity; Selenium; Selenocysteine lyase; Statin.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflicts of interest.
Figures


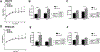
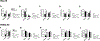
Similar articles
-
Effects of selenium supplementation on diet-induced obesity in mice with a disruption of the selenocysteine lyase gene.J Trace Elem Med Biol. 2020 Dec;62:126596. doi: 10.1016/j.jtemb.2020.126596. Epub 2020 Jul 11. J Trace Elem Med Biol. 2020. PMID: 32683228 Free PMC article.
-
Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice.Mol Cell Biol. 2012 Oct;32(20):4141-54. doi: 10.1128/MCB.00293-12. Epub 2012 Aug 13. Mol Cell Biol. 2012. PMID: 22890841 Free PMC article.
-
Diet-induced obesity in the selenocysteine lyase knockout mouse.Antioxid Redox Signal. 2015 Oct 1;23(10):761-74. doi: 10.1089/ars.2015.6277. Epub 2015 Aug 24. Antioxid Redox Signal. 2015. PMID: 26192035 Free PMC article.
-
Selenium and diabetes--evidence from animal studies.Free Radic Biol Med. 2013 Dec;65:1548-1556. doi: 10.1016/j.freeradbiomed.2013.07.012. Epub 2013 Jul 16. Free Radic Biol Med. 2013. PMID: 23867154 Free PMC article. Review.
-
Selenium, selenoproteins and human health: a review.Public Health Nutr. 2001 Apr;4(2B):593-9. doi: 10.1079/phn2001143. Public Health Nutr. 2001. PMID: 11683552 Review.
Cited by
-
Mechanisms of Ferroptosis and Emerging Links to the Pathology of Neurodegenerative Diseases.Front Aging Neurosci. 2022 Jun 28;14:904152. doi: 10.3389/fnagi.2022.904152. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35837484 Free PMC article. Review.
-
Therapeutic implication of oxidative stress-induced growth inhibitor 1 (OSGIN1) in cancer.Oncogene. 2025 Apr;44(15):997-1006. doi: 10.1038/s41388-025-03349-5. Epub 2025 Mar 17. Oncogene. 2025. PMID: 40097807 Review.
-
Selenium Protects Mouse Hypothalamic Cells from Glucocorticoid-Induced Endoplasmic Reticulum Stress Vulnerability and Insulin Signaling Impairment.Antioxidants (Basel). 2023 Feb 20;12(2):526. doi: 10.3390/antiox12020526. Antioxidants (Basel). 2023. PMID: 36830084 Free PMC article.
-
Intersection between Obesity, Dietary Selenium, and Statin Therapy in Brazil.Nutrients. 2021 Jun 12;13(6):2027. doi: 10.3390/nu13062027. Nutrients. 2021. PMID: 34204631 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

