Azelastine inhibits viropexis of SARS-CoV-2 spike pseudovirus by binding to SARS-CoV-2 entry receptor ACE2
- PMID: 34052578
- PMCID: PMC8144927
- DOI: 10.1016/j.virol.2021.05.009
Azelastine inhibits viropexis of SARS-CoV-2 spike pseudovirus by binding to SARS-CoV-2 entry receptor ACE2
Abstract
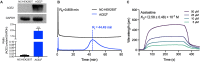
A recent study have reported that pre-use of azelastine is associated with a decrease in COVID-19 positive test results among susceptible elderly people. Besides, it has been reported that antihistamine drugs could prevent viruses from entering cells. The purpose of this study is to investigate whether azelastine have antiviral activity against SARS-CoV-2 in vitro and the possible mechanism. Here, we discovered antihistamine azelastine has an affinity to ACE2 by cell membrane chromatography (CMC); Then we determined the equilibrium dissociation constant (KD) of azelastine-ACE2 as (2.58 ± 0.48) × 10-7 M by surface plasmon resonance (SPR). The results of molecular docking showed that azelastine could form an obvious hydrogen bond with Lys353. The pseudovirus infection experiments showed that azelastine effectively inhibited viral entry (EC50 = 3.834 μM). Our work provides a new perspective for the screening method of drug repositioning for COVID-19, and an attractive and promising drug candidate for anti-SARS-CoV-2.
Keywords: ACE2; Antihistamine; Azelastine; Drug repurposing; SARS-CoV-2.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
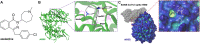
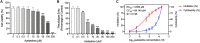
References
-
- Consortium W.H.O.S.T., Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Abdool Karim Q., Alejandria M.M., Hernandez Garcia C., Kieny M.P., Malekzadeh R., Murthy S., Reddy K.S., Roses Periago M., Abi Hanna P., Ader F., Al-Bader A.M., Alhasawi A., Allum E., Alotaibi A., Alvarez-Moreno C.A., Appadoo S., Asiri A., Aukrust P., Barratt-Due A., Bellani S., Branca M., Cappel-Porter H.B.C., Cerrato N., Chow T.S., Como N., Eustace J., Garcia P.J., Godbole S., Gotuzzo E., Griskevicius L., Hamra R., Hassan M., Hassany M., Hutton D., Irmansyah I., Jancoriene L., Kirwan J., Kumar S., Lennon P., Lopardo G., Lydon P., Magrini N., Maguire T., Manevska S., Manuel O., McGinty S., Medina M.T., Mesa Rubio M.L., Miranda-Montoya M.C., Nel J., Nunes E.P., Perola M., Portoles A., Rasmin M.R., Raza A., Rees H., Reges P.P.S., Rogers C.A., Salami K., Salvadori M.I., Sinani N., Sterne J.A.C., Stevanovikj M., Tacconelli E., Tikkinen K.A.O., Trelle S., Zaid H., Rottingen J.A., Swaminathan S. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. - DOI - PMC - PubMed
-
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

