Cathepsin K deficiency promotes alveolar bone regeneration by promoting jaw bone marrow mesenchymal stem cells proliferation and differentiation via glycolysis pathway
- PMID: 34053135
- PMCID: PMC8249792
- DOI: 10.1111/cpr.13058
Cathepsin K deficiency promotes alveolar bone regeneration by promoting jaw bone marrow mesenchymal stem cells proliferation and differentiation via glycolysis pathway
Abstract
Objectives: To clarify the possible role and mechanism of Cathepsin K (CTSK) in alveolar bone regeneration mediated by jaw bone marrow mesenchymal stem cells (JBMMSC).
Materials and methods: Tooth extraction models of Ctsk knockout mice (Ctsk-/- ) and their wildtype (WT) littermates were used to investigate the effect of CTSK on alveolar bone regeneration. The influences of deletion or inhibition of CTSK by odanacatib (ODN) on proliferation and osteogenic differentiation of JBMMSC were assessed by CCK-8, Western blot and alizarin red staining. To explore the differently expressed genes, RNA from WT and Ctsk-/- JBMMSC was sent to RNA-seq. ECAR, glucose consumption and lactate production were measured to identify the effect of Ctsk deficiency or inhibition on glycolysis. At last, we explored whether Ctsk deficiency or inhibition promoted JBMMSC proliferation and osteogenic differentiation through glycolysis.
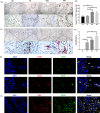
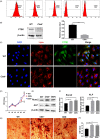
Results: We found out that Ctsk knockout could promote alveolar bone regeneration in vivo. In vitro, we confirmed that both Ctsk knockout and inhibition by ODN could promote proliferation of JBMMSC, up-regulate expression of Runx2 and ALP, and enhance matrix mineralization. RNA-seq results showed that coding genes of key enzymes in glycolysis were significantly up-regulated in Ctsk-/- JBMMSC, and Ctsk deficiency or inhibition could promote glycolysis in JBMMSC. After blocking glycolysis by 3PO, the effect of Ctsk deficiency or inhibition on JBMMSC's regeneration was blocked subsequently.
Conclusions: Our findings revealed that Ctsk knockout or inhibition could promote alveolar bone regeneration by enhancing JBMMSC regeneration via glycolysis. These results shed new lights on the regulatory mechanism of CTSK on bone regeneration.
Keywords: Cathepsin K; alveolar bone; glycolysis; jaw bone marrow mesenchymal stem cells; osteogenic differentiation.
© 2021 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests regarding the publication of this paper.
Figures




Similar articles
-
[Inhibition of osteogenic differentiation of mouse bone marrow mesenchymal stem cells and maxillary expansion osteogenesis by cytoskeleton-associated protein 4 knockout].Zhonghua Kou Qiang Yi Xue Za Zhi. 2025 May 9;60(5):525-533. doi: 10.3760/cma.j.cn112144-20241009-00376. Zhonghua Kou Qiang Yi Xue Za Zhi. 2025. PMID: 40268538 Chinese.
-
The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets.Biomaterials. 2013 Dec;34(38):9937-51. doi: 10.1016/j.biomaterials.2013.09.017. Epub 2013 Oct 1. Biomaterials. 2013. PMID: 24095254
-
Adiponectin promotes human jaw bone marrow mesenchymal stem cell chemotaxis via CXCL1 and CXCL8.J Cell Mol Med. 2017 Jul;21(7):1411-1419. doi: 10.1111/jcmm.13070. Epub 2017 Feb 8. J Cell Mol Med. 2017. PMID: 28176455 Free PMC article.
-
[Research progress in biological characteristics and influencing factors of jaw bone marrow mesenchymal stem cell].Zhonghua Kou Qiang Yi Xue Za Zhi. 2022 Jan 9;57(1):107-112. doi: 10.3760/cma.j.cn112144-20211009-00457. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022. PMID: 35012260 Review. Chinese.
-
Cranial Suture Mesenchymal Stem Cells: Insights and Advances.Biomolecules. 2021 Jul 31;11(8):1129. doi: 10.3390/biom11081129. Biomolecules. 2021. PMID: 34439795 Free PMC article. Review.
Cited by
-
Cathepsin K in Pathological Conditions and New Therapeutic and Diagnostic Perspectives.Int J Mol Sci. 2022 Nov 9;23(22):13762. doi: 10.3390/ijms232213762. Int J Mol Sci. 2022. PMID: 36430239 Free PMC article. Review.
-
Recent Advances in Single-Cell View of Mesenchymal Stem Cell in Osteogenesis.Front Cell Dev Biol. 2022 Jan 5;9:809918. doi: 10.3389/fcell.2021.809918. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35071243 Free PMC article. Review.
-
Adipokines regulate mesenchymal stem cell osteogenic differentiation.World J Stem Cells. 2023 Jun 26;15(6):502-513. doi: 10.4252/wjsc.v15.i6.502. World J Stem Cells. 2023. PMID: 37424950 Free PMC article. Review.
-
The effects of Twinlight laser treatment on the titanium surface proliferation and osteogenic differentiation of mesenchymal stem cells.BMC Oral Health. 2022 Sep 19;22(1):409. doi: 10.1186/s12903-022-02448-z. BMC Oral Health. 2022. PMID: 36123683 Free PMC article.
-
Treatment with an inhibitor of matrix metalloproteinase 9 or cathepsin K lengthens embryonic lower jaw bone.Orthod Craniofac Res. 2023 Aug;26(3):500-509. doi: 10.1111/ocr.12635. Epub 2023 Feb 1. Orthod Craniofac Res. 2023. PMID: 36680416 Free PMC article.
References
-
- Delaissé JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech. 2003;61(6):504‐513. - PubMed
-
- Troen BR. The role of cathepsin K in normal bone resorption. Drug News Perspect. 2004;17(1):19‐28. - PubMed
-
- Liu F, Zhou ZF, An Y, et al. Effects of cathepsin K on Emdogain‐induced hard tissue formation by human periodontal ligament stem cells. J Tissue Eng Regen Med. 2017;11(10):2922‐2934. - PubMed
-
- Whitty C, Wardale RJ, Henson FMD. The regulation of sclerostin by cathepsin K in periodontal ligament cells. Biochem Biophys Res Commun. 2018;503(2):550‐555. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

