Proteoglycan synthesis in conserved oligomeric Golgi subunit deficient HEK293T cells is affected differently, depending on the lacking subunit
- PMID: 34053170
- PMCID: PMC8382154
- DOI: 10.1111/tra.12804
Proteoglycan synthesis in conserved oligomeric Golgi subunit deficient HEK293T cells is affected differently, depending on the lacking subunit
Abstract
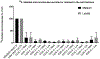
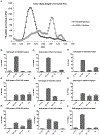
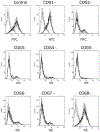
The Conserved Oligomeric Golgi (COG) complex is an eight subunit protein complex associated with Golgi membranes. Genetic defects affecting individual COG subunits cause congenital disorders of glycosylation (CDGs), due to mislocalization of Golgi proteins involved in glycosylation mechanisms. While the resulting defects in N-and O-glycosylation have been extensively studied, no corresponding study of proteoglycan (PG) synthesis has been undertaken. We here show that glycosaminoglycan (GAG) modification of PGs is significantly reduced, regardless which COG subunit that is missing in HEK293T cells. Least reduction was observed for cells lacking COG1 and COG8 subunits, that bridge the A and B lobes of the complex. Lack of these subunits did not reduce GAG chain lengths of secreted PGs, which was reduced in cells lacking any other subunit (COG2-7). COG3 knock out (KO) cells had particularly reduced ability to polymerize GAG chains. For cell-associated GAGs, the mutant cell lines, except COG4 and COG7 KO, displayed longer GAG chains than wild-type cells, indicating that COG subunits play a role in cellular turnover of PGs. In light of the important roles PGs play in animal development, the effects KO of individual COG subunits have on GAG synthesis could explain the variable severity of COG associated CDGs.
Keywords: congenital disorders of glycosylation; glycosaminoglycan; glycosylation; proteoglycans; the Golgi apparatus; the conserved oligomeric complex.
© 2021 The Authors. Traffic published by John Wiley & Sons Ltd.
Figures



References
-
- Miller VJ, Ungar D. Re’COG’nition at the Golgi. Traffic 2012; 13(7): 891–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

