Cardiac metallothionein overexpression rescues diabetic cardiomyopathy in Akt2-knockout mice
- PMID: 34053181
- PMCID: PMC8278119
- DOI: 10.1111/jcmm.16687
Cardiac metallothionein overexpression rescues diabetic cardiomyopathy in Akt2-knockout mice
Abstract
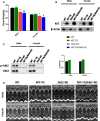
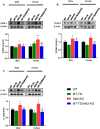
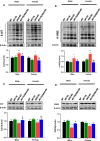
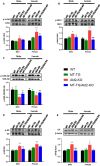
To efficiently prevent diabetic cardiomyopathy (DCM), we have explored and confirmed that metallothionein (MT) prevents DCM by attenuating oxidative stress, and increasing expression of proteins associated with glucose metabolism. To determine whether Akt2 expression is critical to MT prevention of DCM, mice with either global Akt2 gene deletion (Akt2-KO), or cardiomyocyte-specific overexpressing MT gene (MT-TG) or both combined (MT-TG/Akt2-KO) were used. Akt2-KO mice exhibited symptoms of DCM (cardiac remodelling and dysfunction), and reduced expression of glycogen and glucose metabolism-related proteins, despite an increase in total Akt (t-Akt) phosphorylation. Cardiac MT overexpression in MT-TG/Akt2-KO mice prevented DCM and restored glucose metabolism-related proteins expression and baseline t-Akt phosphorylation. Furthermore, phosphorylation of ERK1/2 increased in the heart of MT-TG/Akt2-KO mice, compared with Akt2-KO mice. As ERK1/2 has been implicated in the regulation of glucose transport and metabolism this increase could potentially underlie MT protective effect in MT-TG/Akt2-KO mice. Therefore, these results show that although our previous work has shown that MT preserving Akt2 activity is sufficient to prevent DCM, in the absence of Akt2 MT may stimulate alternative or downstream pathways protecting from DCM in a type 2 model of diabetes, and that this protection may be associated with the ERK activation pathway.
Keywords: Akt2 knock out; diabetes; glucose metabolism; insulin resistance; metallothionein.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Diabetic cardiomyopathy - Zinc preventive and therapeutic potentials by its anti-oxidative stress and sensitizing insulin signaling pathways.Toxicol Appl Pharmacol. 2023 Oct 15;477:116694. doi: 10.1016/j.taap.2023.116694. Epub 2023 Sep 20. Toxicol Appl Pharmacol. 2023. PMID: 37739320 Free PMC article. Review.
-
Metallothionein Preserves Akt2 Activity and Cardiac Function via Inhibiting TRB3 in Diabetic Hearts.Diabetes. 2018 Mar;67(3):507-517. doi: 10.2337/db17-0219. Epub 2017 Oct 27. Diabetes. 2018. PMID: 29079702 Free PMC article.
-
Zinc rescue of Akt2 gene deletion-linked murine cardiac dysfunction and pathological changes is metallothionein-dependent.J Mol Cell Cardiol. 2014 Sep;74:88-97. doi: 10.1016/j.yjmcc.2014.04.023. Epub 2014 May 10. J Mol Cell Cardiol. 2014. PMID: 24819347
-
Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein.Free Radic Biol Med. 2017 Nov;112:224-239. doi: 10.1016/j.freeradbiomed.2017.07.031. Epub 2017 Aug 2. Free Radic Biol Med. 2017. PMID: 28778483 Free PMC article.
-
Diabetic cardiomyopathy and its prevention by metallothionein: experimental evidence, possible mechanisms and clinical implications.Curr Med Chem. 2007;14(20):2193-203. doi: 10.2174/092986707781389646. Curr Med Chem. 2007. PMID: 17691957 Review.
Cited by
-
New insights into the role of metallothioneins in obesity and diabetes.Int J Obes (Lond). 2025 Jul 14. doi: 10.1038/s41366-025-01850-1. Online ahead of print. Int J Obes (Lond). 2025. PMID: 40659851 Review.
-
Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies.Biomedicines. 2023 Apr 7;11(4):1126. doi: 10.3390/biomedicines11041126. Biomedicines. 2023. PMID: 37189744 Free PMC article. Review.
-
The role of Zn2+ in shaping intracellular Ca2+ dynamics in the heart.J Gen Physiol. 2023 Jul 3;155(7):e202213206. doi: 10.1085/jgp.202213206. Epub 2023 Jun 16. J Gen Physiol. 2023. PMID: 37326614 Free PMC article. Review.
-
The Molecular Mechanisms of Defective Copper Metabolism in Diabetic Cardiomyopathy.Oxid Med Cell Longev. 2022 Oct 4;2022:5418376. doi: 10.1155/2022/5418376. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36238639 Free PMC article. Review.
-
Diabetic cardiomyopathy - Zinc preventive and therapeutic potentials by its anti-oxidative stress and sensitizing insulin signaling pathways.Toxicol Appl Pharmacol. 2023 Oct 15;477:116694. doi: 10.1016/j.taap.2023.116694. Epub 2023 Sep 20. Toxicol Appl Pharmacol. 2023. PMID: 37739320 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

