Treadmill Running Improves Spatial Learning Memory Through Inactivation of Nuclear Factor Kappa B/Mitogen-Activated Protein Kinase Signaling Pathway in Amyloid-β-Induced Alzheimer Disease Rats
- PMID: 34053209
- PMCID: PMC8171239
- DOI: 10.5213/inj.2142164.082
Treadmill Running Improves Spatial Learning Memory Through Inactivation of Nuclear Factor Kappa B/Mitogen-Activated Protein Kinase Signaling Pathway in Amyloid-β-Induced Alzheimer Disease Rats
Abstract
Purpose: Exercise is known to reduce proinflammatory cytokines production and apoptosis. We investigated the effect of treadmill running on spatial learning memory in terms of activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathway in Alzheimer disease (AD) rats. We also evaluated the effect of treadmill running on proinflammatory cytokine production and apoptosis.
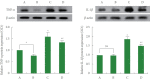
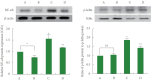
Methods: Using the stereotaxic frame, amyloid-β (Aβ) was injected into the lateral ventricle of the brain. The rats belong to treadmill running groups were forced to run on a motorized treadmill for 30 minutes per a day during 4 weeks, starting 3 days after Aβ injection. Morris water maze task was done for the determination of spatial learning memory. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, immunohistochemistry for cleaved caspase-3, and western blot for NF-κB, inhibitory protein of NF-κB (IκB), MAPK signaling pathway, tumor necrosis factor (TNF)-α, interleukin (IL)-1β were done.
Results: Induction of AD increased proinflammatory cytokine secretion by activating the NF-κB/MAPK signaling pathway. These changes induced apoptosis in the hippocampus and reduced spatial learning memory. In contrast, treadmill running inactivated the NF-κB/MAPK signaling pathway and suppressed proinflammatory cytokine production. These changes inhibited apoptosis and improved spatial learning memory.
Conclusion: Current results showed that treadmill running promoted spatial learning memory through suppressing proinflammatory cytokine production and apoptosis via inactivation of NF-κB/MAPK signaling pathway. Treadmill exercise can be considered an effective intervention for symptom relieve of AD.
Keywords: Alzheimer disease; Amyloid-β; Apoptosis; Inflammation; Treadmill exercise.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



Similar articles
-
Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in β-amyloid rat model of Alzheimer's disease.J Neuroinflammation. 2012 Aug 17;9:202. doi: 10.1186/1742-2094-9-202. J Neuroinflammation. 2012. PMID: 22898621 Free PMC article.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Voluntary Wheel Running Improves Spatial Learning Memory by Suppressing Inflammation and Apoptosis via Inactivation of Nuclear Factor Kappa B in Brain Inflammation Rats.Int Neurourol J. 2020 Nov;24(Suppl 2):96-103. doi: 10.5213/inj.2040432.216. Epub 2020 Nov 23. Int Neurourol J. 2020. PMID: 33271006 Free PMC article.
-
Hyperbaric Oxygen Pretreatment Improves Cognition and Reduces Hippocampal Damage Via p38 Mitogen-Activated Protein Kinase in a Rat Model.Yonsei Med J. 2017 Jan;58(1):131-138. doi: 10.3349/ymj.2017.58.1.131. Yonsei Med J. 2017. PMID: 27873505 Free PMC article.
-
Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats.J Exerc Rehabil. 2018 Jun 30;14(3):327-334. doi: 10.12965/jer.1836248.124. eCollection 2018 Jun. J Exerc Rehabil. 2018. PMID: 30018914 Free PMC article.
Cited by
-
Exercise training exerts beneficial effects on Alzheimer's disease through multiple signaling pathways.Front Aging Neurosci. 2025 May 21;17:1558078. doi: 10.3389/fnagi.2025.1558078. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40469843 Free PMC article. Review.
-
Positive effects of HIIT and vitamin E on cognitive function, angiogenesis and NRF2 antioxidant pathway in male rats with Alzheimer's disease.Biogerontology. 2025 Jun 25;26(4):129. doi: 10.1007/s10522-025-10274-3. Biogerontology. 2025. PMID: 40562949
-
Early exercise intervention promotes myelin repair in the brains of ischemic rats by inhibiting the MEK/ERK pathway.Transl Neurosci. 2024 Mar 14;15(1):20220335. doi: 10.1515/tnsci-2022-0335. eCollection 2024 Jan 1. Transl Neurosci. 2024. PMID: 38511170 Free PMC article.
-
Functional Mechanism of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Animal Models with Alzheimer's Disease: Inhibition of Neuroinflammation.J Inflamm Res. 2021 Sep 17;14:4761-4775. doi: 10.2147/JIR.S327538. eCollection 2021. J Inflamm Res. 2021. PMID: 34566422 Free PMC article. Review.
-
Strategies for Overcoming Intractable Disorders or Memory Impairments.Int Neurourol J. 2021 May;25(Suppl 1):S1-2. doi: 10.5213/inj.2121edi.002. Epub 2021 May 31. Int Neurourol J. 2021. PMID: 34053204 Free PMC article. No abstract available.
References
-
- Agnihotri A, Aruoma OI. Alzheimer’s disease and Parkinson’s disease: a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental chemicals. J Am Coll Nutr. 2020;39:16–27. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

