Biallelic and monoallelic variants in PLXNA1 are implicated in a novel neurodevelopmental disorder with variable cerebral and eye anomalies
- PMID: 34054129
- PMCID: PMC8460429
- DOI: 10.1038/s41436-021-01196-9
Biallelic and monoallelic variants in PLXNA1 are implicated in a novel neurodevelopmental disorder with variable cerebral and eye anomalies
Abstract
Purpose: To investigate the effect of PLXNA1 variants on the phenotype of patients with autosomal dominant and recessive inheritance patterns and to functionally characterize the zebrafish homologs plxna1a and plxna1b during development.
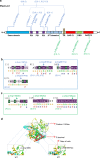
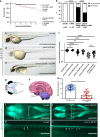
Methods: We assembled ten patients from seven families with biallelic or de novo PLXNA1 variants. We describe genotype-phenotype correlations, investigated the variants by structural modeling, and used Morpholino knockdown experiments in zebrafish to characterize the embryonic role of plxna1a and plxna1b.
Results: Shared phenotypic features among patients include global developmental delay (9/10), brain anomalies (6/10), and eye anomalies (7/10). Notably, seizures were predominantly reported in patients with monoallelic variants. Structural modeling of missense variants in PLXNA1 suggests distortion in the native protein. Our zebrafish studies enforce an embryonic role of plxna1a and plxna1b in the development of the central nervous system and the eye.
Conclusion: We propose that different biallelic and monoallelic variants in PLXNA1 result in a novel neurodevelopmental syndrome mainly comprising developmental delay, brain, and eye anomalies. We hypothesize that biallelic variants in the extracellular Plexin-A1 domains lead to impaired dimerization or lack of receptor molecules, whereas monoallelic variants in the intracellular Plexin-A1 domains might impair downstream signaling through a dominant-negative effect.
© 2021. The Author(s).
Conflict of interest statement
J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals and Novartis and is a member of the Scientific Advisory Board of Baylor Genetics, and is a co-inventor on multiple US and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis (CMA) and clinical exome sequencing offered at Baylor Genetics. S.T.C. is director of Frontier Genomics Pty Ltd (Australia). Frontier Genomics has not traded (as of December 2019). Frontier Genomics Pty Ltd (Australia) has no existing financial relationships that will benefit from publication of these data. R.E.S. is an employee of GeneDx, Inc. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

