Acute Bacterial Tenonitis and Conjunctivitis following Intravitreal Injection
- PMID: 34054476
- PMCID: PMC8138147
- DOI: 10.1159/000511862
Acute Bacterial Tenonitis and Conjunctivitis following Intravitreal Injection
Abstract
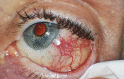
A 73-year-old man presented 3 days after intravitreal injection (IVI) with bevacizumab for treatment of neovascular age-related macular degeneration with pain and redness around the injection site. Examination showed conjunctival edema and injection around the injection site and a central infiltrate at the injection site consistent with infection of Tenon's capsule and the conjunctiva. Infection of a vitreous wick was considered, but vitreous inflammation was not present. Acute bacterial tenonitis and conjunctivitis were diagnosed, and the patient was prescribed topical antibiotic drops. The patient's symptoms were resolved within 48 h following the use of topical antibiotic drops, so a culture was not performed. The patient did not develop endophthalmitis. To our knowledge, this is the first reported case of acute bacterial tenonitis and conjunctivitis of the injection site following IVI. Even with the use of betadine, infection of Tenon's capsule and the conjunctiva may occur after IVI and must be differentiated from other causes of postinjection ocular redness such as chemical irritation of the ocular surface, corneal abrasions, and endophthalmitis.
Keywords: Conjunctivitis; Intravitreal injection; Ocular redness; Tenonitis.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to share.
Figures
References
-
- Daien V, Nguyen V, Essex RW, Morlet N, Barthelmes D, Gillies MC, et al. Fight Retinal Blindness! Study Group Incidence and Outcomes of Infectious and Noninfectious Endophthalmitis after Intravitreal Injections for Age-Related Macular Degeneration. Ophthalmology. 2018 Jan;125((1)):66–74. - PubMed
-
- Ha D, Choi S-R, Kwon Y, Park H-H, Shin J-Y. Pattern of adverse events induced by aflibercept and ranibizumab A nationwide spontaneous adverse event reporting database, 2007-2016. 2019 Available from: http://dx.doi.org/ - PMC - PubMed
-
- Epling J. BMJ clinical evidence. Vol. 2012. BMJ Publishing Group; 2012. Bacterial conjunctivitis. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources