Implications for Extracellular Matrix Interactions With Human Lung Basal Stem Cells in Lung Development, Disease, and Airway Modeling
- PMID: 34054525
- PMCID: PMC8149957
- DOI: 10.3389/fphar.2021.645858
Implications for Extracellular Matrix Interactions With Human Lung Basal Stem Cells in Lung Development, Disease, and Airway Modeling
Abstract
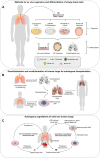
The extracellular matrix (ECM) is not simply a quiescent scaffold. This three-dimensional network of extracellular macromolecules provides structural, mechanical, and biochemical support for the cells of the lung. Throughout life, the ECM forms a critical component of the pulmonary stem cell niche. Basal cells (BCs), the primary stem cells of the airways capable of differentiating to all luminal cell types, reside in close proximity to the basolateral ECM. Studying BC-ECM interactions is important for the development of therapies for chronic lung diseases in which ECM alterations are accompanied by an apparent loss of the lung's regenerative capacity. The complexity and importance of the native ECM in the regulation of BCs is highlighted as we have yet to create an in vitro culture model that is capable of supporting the long-term expansion of multipotent BCs. The interactions between the pulmonary ECM and BCs are, therefore, a vital component for understanding the mechanisms regulating BC stemness during health and disease. If we are able to replicate these interactions in airway models, we could significantly improve our ability to maintain basal cell stemness ex vivo for use in in vitro models and with prospects for cellular therapies. Furthermore, successful, and sustained airway regeneration in an aged or diseased lung by small molecules, novel compounds or via cellular therapy will rely upon both manipulation of the airway stem cells and their immediate niche within the lung. This review will focus on the current understanding of how the pulmonary ECM regulates the basal stem cell function, how this relationship changes in chronic disease, and how replicating native conditions poses challenges for ex vivo cell culture.
Keywords: airway modeling; basal stem cells; cellular niche; chronic lung disease; extracellular matrix; regeneration.
Copyright © 2021 Busch, Lorenzana and Ryan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

