Non-Peptidic Small Molecule Components from Cone Snail Venoms
- PMID: 34054536
- PMCID: PMC8155685
- DOI: 10.3389/fphar.2021.655981
Non-Peptidic Small Molecule Components from Cone Snail Venoms
Abstract
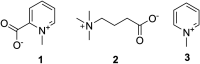
Venomous molluscs (Superfamily Conoidea) comprise a substantial fraction of tropical marine biodiversity (>15,000 species). Prior characterization of cone snail venoms established that bioactive venom components used to capture prey, defend against predators and for competitive interactions were relatively small, structured peptides (10-35 amino acids), most with multiple disulfide crosslinks. These venom components ("conotoxins, conopeptides") have been widely studied in many laboratories, leading to pharmaceutical agents and probes. In this review, we describe how it has recently become clear that to varying degrees, cone snail venoms also contain bioactive non-peptidic small molecule components. Since the initial discovery of genuanine as the first bioactive venom small molecule with an unprecedented structure, a broad set of cone snail venoms have been examined for non-peptidic bioactive components. In particular, a basal clade of cone snails (Stephanoconus) that prey on polychaetes produce genuanine and many other small molecules in their venoms, suggesting that this lineage may be a rich source of non-peptidic cone snail venom natural products. In contrast to standing dogma in the field that peptide and proteins are predominantly used for prey capture in cone snails, these small molecules also contribute to prey capture and push the molecular diversity of cone snails beyond peptides. The compounds so far characterized are active on neurons and thus may potentially serve as leads for neuronal diseases. Thus, in analogy to the incredible pharmacopeia resulting from studying venom peptides, these small molecules may provide a new resource of pharmacological agents.
Keywords: conopeptides; conus; gastropod; natural products; nicotinic acetylcholine receptor; prey capture; secondary metabolites; venom.
Copyright © 2021 Lin, Torres, Watkins, Paguigan, Niu, Imperial, Tun, Safavi-Hemami, Finol-Urdaneta, Neves, Espino, Karthikeyan, Olivera and Schmidt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abalde S., Tenorio M. J., Uribe J. E., Zardoya R. (2019). Conidae phylogenomics and evolution. Zool Scr 48, 194–214. 10.1111/zsc.12329 - DOI
-
- Aknin M., Viracaoundin I., Faure R., Gaydou E. M. (1998). 5α,8α-Epidioxycholest-6-en-3-β-ol from three cone snails of the Indian ocean. J. Amer Oil Chem. Soc. 75, 1679–1681. 10.1007/s11746-998-0111-y - DOI
-
- Arbiser J. L., Nowak R., Michaels K., Skabytska Y., Biedermann T., Lewis M. J., et al. (2017). Evidence for biochemical barrier restoration: topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis. Sci. Rep. 7 (1), 11198. 10.1038/s41598-017-10580-y - DOI - PMC - PubMed
-
- Arnison P. G., Bibb M. J., Bierbaum G., Bowers A. A., Bugni T. S., Bulaj G., et al. (2013). Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30 (1), 108–160. 10.1039/c2np20085f - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

