Pharmacological Vitamin C Treatment Impedes the Growth of Endogenous Glutamine-Dependent Cancers by Targeting Glutamine Synthetase
- PMID: 34054545
- PMCID: PMC8150514
- DOI: 10.3389/fphar.2021.671902
Pharmacological Vitamin C Treatment Impedes the Growth of Endogenous Glutamine-Dependent Cancers by Targeting Glutamine Synthetase
Abstract
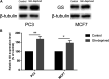
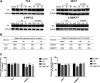
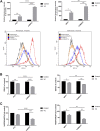
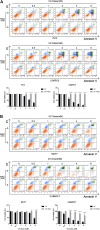
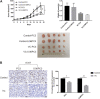
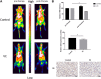
Purpose: Glutamine synthetase (GS) is the only currently known enzyme responsible for synthesizing endogenous glutamine (Gln). GS exerts a critical role in the oncogenesis of endogenous Gln-dependent cancers, making it an attractive target for anti-tumor therapies. A mixed-function oxidation system consisting of vitamin C (VC), oxygen, and trace metals can oxidize GS and promote its degradation. The current study aims to explore the effect of pharmacological VC treatment on GS. Methods: Endogenous Gln-dependent cancer lines (breast cancer MCF7 and prostate cancer PC3) were selected to establish chronic Gln-deprived MCF7 and PC3 cell models. The expression of GS in parental and chronic Gln-deprived tumor cells exposed to VC treatment and control was determined by Western blot analysis. The anti-cancer effects of VC on parental and chronic Gln-deprived tumor cells were assessed by CCK-8 and annexin V-FITC/PI FACS assays. In addition, changes in cellular reactive oxygen species (ROS), glutathione (GSH) levels and NADPH/NADP + ratio were analyzed to explore the underlying mechanisms. Moreover, BALB/c nude mice xenografting with parental and chronic Gln-deprived prostate cancer cells were constructed to evaluate the in vivo therapeutic effect of VC. Finally, tumor 13N-ammonia uptake in mice bearing prostate cancer xenografts was analyzed following treatment with VC and the expression of GS in xenografts were detected by immunohistochemistry. Results: Cells overexpressing GS were obtained by chronic Gln deprivation. We found that the cytotoxic effect of VC on cancer cells was positively correlated with the expression of GS. Additionally, VC treatment led to a significant increase in ROS production, as well as GSH depletion and NADPH/NADP + reduction. These changes could be reversed by the antioxidant N-acetyl-L-cysteine (NAC). Furthermore, pharmacological VC treatment exhibited a more significant therapeutic effect on xenografts of prostate cancer cells overexpressing GS, that could be well monitored by 13N-ammonia PET/CT imaging. Conclusion: Our findings indicate that VC can kill cancer cells by targeting glutamine synthetase to induce oxidative stress. VC could be used as an anti-cancer treatment for endogenous glutamine-dependent cancers.
Keywords: 13N-ammonia PET/CT; endogenous glutamine-dependent cancer; glutamine synthetase; redox stress; vitamin C.
Copyright © 2021 Long, Qiu, Zhang, He, Shi, He, Chen, Shen, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Xc- inhibitor sulfasalazine improves the anti-cancer effect of pharmacological vitamin C in prostate cancer cells via a glutathione-dependent mechanism.Cell Oncol (Dordr). 2020 Feb;43(1):95-106. doi: 10.1007/s13402-019-00474-8. Epub 2019 Oct 15. Cell Oncol (Dordr). 2020. PMID: 31617161
-
De Novo Glutamine Synthesis: Importance for the Proliferation of Glioma Cells and Potentials for Its Detection With 13N-Ammonia.Mol Imaging. 2016 Apr 26;15:1536012116645440. doi: 10.1177/1536012116645440. Print 2016. Mol Imaging. 2016. PMID: 27118759 Free PMC article.
-
Targeting of glutamine transporter ASCT2 and glutamine synthetase suppresses gastric cancer cell growth.J Cancer Res Clin Oncol. 2018 May;144(5):821-833. doi: 10.1007/s00432-018-2605-9. Epub 2018 Feb 12. J Cancer Res Clin Oncol. 2018. PMID: 29435734 Free PMC article.
-
Glutamine Synthetase as a Therapeutic Target for Cancer Treatment.Int J Mol Sci. 2021 Feb 8;22(4):1701. doi: 10.3390/ijms22041701. Int J Mol Sci. 2021. PMID: 33567690 Free PMC article. Review.
-
Glutamine Synthetase: Localization Dictates Outcome.Genes (Basel). 2018 Feb 19;9(2):108. doi: 10.3390/genes9020108. Genes (Basel). 2018. PMID: 29463059 Free PMC article. Review.
Cited by
-
Strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy.J Transl Med. 2024 Aug 9;22(1):751. doi: 10.1186/s12967-024-05552-6. J Transl Med. 2024. PMID: 39123227 Free PMC article. Review.
-
Small molecule inhibitors for cancer metabolism: promising prospects to be explored.J Cancer Res Clin Oncol. 2023 Aug;149(10):8051-8076. doi: 10.1007/s00432-022-04501-4. Epub 2023 Mar 31. J Cancer Res Clin Oncol. 2023. PMID: 37002510 Free PMC article. Review.
-
Enhanced Antitumor Effect in Liver Cancer by Amino Acid Depletion-Induced Oxidative Stress.Front Oncol. 2021 Nov 2;11:758549. doi: 10.3389/fonc.2021.758549. eCollection 2021. Front Oncol. 2021. PMID: 34796113 Free PMC article.
-
Targeting Glutamine Metabolism in Prostate Cancer.Front Biosci (Elite Ed). 2023 Jan 4;15(1):2. doi: 10.31083/j.fbe1501002. Front Biosci (Elite Ed). 2023. PMID: 36959101 Free PMC article. Review.
-
Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment.Cancers (Basel). 2022 May 25;14(11):2608. doi: 10.3390/cancers14112608. Cancers (Basel). 2022. PMID: 35681589 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

