Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation
- PMID: 34054546
- PMCID: PMC8160516
- DOI: 10.3389/fphar.2021.672054
Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation
Abstract
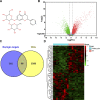
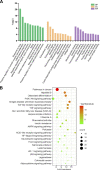
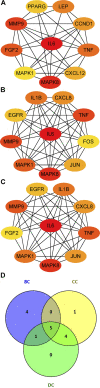
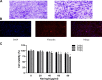
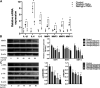
Rheumatoid arthritis is a chronic autoimmune disease characterized by persistent hyperplasia of the synovial membrane and progressive erosion of articular cartilage. Disequilibrium between the proliferation and death of RA fibroblast-like synoviocytes (RA-FLSs) is the critical factor in progression of RA. Naringin has been reported to exert anti-inflammatory and antioxidant effect in acute and chronic animal models of RA. However, the therapeutic effect and underlying mechanisms of naringin in human RA-FLS remain unclear. Based on network pharmacology, the corresponding targets of naringin were identified using SwissTargetPrediction database, STITCH database, and Comparative Toxicogenomics Database. Deferentially expressed genes (DEGs) in RA were obtained from the GEO database. The protein-protein interaction (PPI) networks of intersected targets were constructed using the STRING database and visualized using Cytoscape. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed, and the pathways directly related to pathogenesis of RA were integrated manually. Further, in vitro studies were carried out based on network pharmacology. 99 target genes were intersected between targets of naringin and DEGs. The PPI network and topological analysis indicated that IL-6, MAPK8, MMP-9, TNF, and MAPK1 shared the highest centrality among all. GO analysis and KEGG analysis indicated that target genes were mostly enriched in (hsa05200) pathways in cancer, (hsa05161) hepatitis B, (hsa04380) osteoclast differentiation, (hsa04151) PI3K-Akt signaling pathway, and (hsa05142) Chagas disease (American trypanosomiasis). In vitro studies revealed that naringin exposure was found to promote apoptosis of RA-FLS, increased the activation of caspase-3, and increased the ratio of Bax/Bcl-2 in a dose-dependent manner. Furthermore, treatment of naringin attenuated the production of inflammatory cytokines and matrix metalloproteinases (MMPs) in TNF-ɑ-induced RA-FLS. Moreover, treatment of naringin inhibited the phosphorylation of Akt and ERK in RA-FLS. Network pharmacology provides a predicative strategy to investigate the therapeutic effects and mechanisms of herbs and compounds. Naringin inhibits inflammation and MMPs production and promotes apoptosis in RA-FLS via PI3K/Akt and MAPK/ERK signaling pathways.
Keywords: MAPK/ERK signaling pathway; PI3k/Akt signaling pathway; naringin; network pharmacology; rheumatoid arthritis.
Copyright © 2021 Aihaiti, Song Cai, Tuerhong, Ni Yang, Ma, Shi Zheng, Xu and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Aroui S., Aouey B., Chtourou Y., Meunier A-C., Fetoui H., Kenani A. (2016). Naringin Suppresses Cell Metastasis and the Expression of Matrix Metalloproteinases (MMP-2 and MMP-9) via the Inhibition of ERK-P38-JNK Signaling Pathway in Human Glioblastoma. Chemico-Biological Interactions 244, 195–203. 10.1016/j.cbi.2015.12.011 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

