Inflammatory Immune Cytokine TNF-α Modulates Ezrin Protein Activation via FAK/RhoA Signaling Pathway in PMVECs Hyperpermeability
- PMID: 34054551
- PMCID: PMC8152434
- DOI: 10.3389/fphar.2021.676817
Inflammatory Immune Cytokine TNF-α Modulates Ezrin Protein Activation via FAK/RhoA Signaling Pathway in PMVECs Hyperpermeability
Abstract
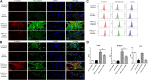
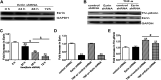
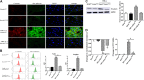
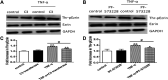
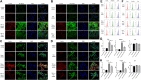
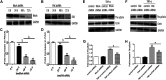
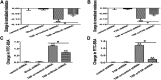
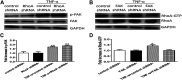
Background: One of the important pathogenesis of acute respiratory distress syndrome (ARDS) is the dysfunction of pulmonary microvascular endothelial barrier induced by a hyperinflammatory immune response. However, the potential mechanisms of such an imbalance in pulmonary microvascular endothelial cells (PMVECs) are not yet understood. Purpose: Explore the molecular mechanism of endothelial barrier dysfunction induced by inflammatory immune cytokines in ARDS, and find a therapeutic target for this syndrome. Methods: Rat PMVECs were cultured to form a monolayer. Immunofluorescence, flow cytometry, and Western blotting were selected to detect the distribution and the expression level of phosphorylated Ezrin protein and Ezrin protein. Transendothelial electrical resistance (TER) and transendothelial fluxes of fluorescein isothiocyanate (FITC)-labeled bovine serum albumin (BSA) were utilized to measure the permeability of the cell monolayer. Ezrin short hairpin RNA (shRNA) and Ezrin 567-site threonine mutant (EzrinT567A) were used to examine the role of Ezrin protein and phosphorylated Ezrin protein in endothelial response induced by tumor necrosis factor-alpha (TNF-α), respectively. The function of focal adhesion kinase (FAK) and Ras homolog gene family, member A (RhoA) signaling pathways were estimated by inhibitors and RhoA/FAK shRNA in TNF-α-stimulated rat PMVECs. The activation of FAK and RhoA was assessed by Western blotting or pull-down assay plus Western blotting. Results: The TER was decreased after TNF-α treatment, while the Ezrin protein phosphorylation was increased in a time- and dose-dependent manner. The phosphorylated Ezrin protein was localized primarily at the cell periphery, resulting in filamentous actin (F-actin) rearrangement, followed by a significant decrease in TER and increase in fluxes of FITC-BSA. Moreover, FAK and RhoA signaling pathways were required in the phosphorylation of Ezrin protein, and the former positively regulated the latter. Conclusion: The phosphorylated Ezrin protein was induced by TNF-α via the FAK/RhoA signaling pathway leading to endothelial hyperpermeability in PMVECs.
Keywords: Ezrin protein; acute respiratory distress syndrome; endothelial permeability; inflammatory immune response; molecular mechanism.
Copyright © 2021 Zhou, Jiang, Chen, Qian and Sun.
Conflict of interest statement
The handling editor declared a shared affiliation with the authors at time of review. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adderley S. P., Lawrence C., Madonia E., Olubadewo J. O., Breslin J. W. (2015). Histamine activates p38 MAP kinase and alters local lamellipodia dynamics, reducing endothelial barrier integrity and eliciting central movement of actin fibers. Am. J. Physiology-Cell Physiol. 309 (1), C51–C59. 10.1152/ajpcell.00096.2015 - DOI - PMC - PubMed
-
- Adyshev D. M., Moldobaeva N. K., Elangovan V. R., Garcia J. G. N., Dudek S. M. (2011). Differential involvement of Ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell Signal. 23 (12), 2086–2096. 10.1016/j.cellsig.2011.08.003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

