In vitro Anticancer Effects of JI017 on Two Prostate Cancer Cell Lines Involve Endoplasmic Reticulum Stress Mediated by Elevated Levels of Reactive Oxygen Species
- PMID: 34054558
- PMCID: PMC8155384
- DOI: 10.3389/fphar.2021.683575
In vitro Anticancer Effects of JI017 on Two Prostate Cancer Cell Lines Involve Endoplasmic Reticulum Stress Mediated by Elevated Levels of Reactive Oxygen Species
Abstract
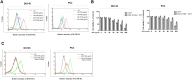
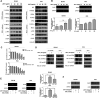
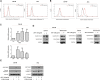
Prostate cancer is the second most commonly diagnosed cancer, and prostate cancer is the second most common cause of cancer death in United States men after lung cancer. Many therapies are used to treat prostate cancer, and chemotherapy is one of the most relevant treatments. However, chemotherapy has many side effects, and repeated administration of chemotherapeutic agents leads to acquired resistance. Thus, new drugs with few side effects are needed. We investigated the molecular mechanism of action of JI017 in human prostate cancer cells. We identified an endoplasmic reticulum (ER) stress pathway that depended on the reactive oxygen species (ROS) pathway and played a crucial role in JI017-induced apoptosis. We measured cell viability by the MTS assay to determine the effect of JI017. Analysis of apoptosis, mitochondrial dysfunction, and cell cycle features was performed by flow cytometry. We used western blot and RT-PCR to measure the levels of the proteins of the unfolded protein response (UPR) pathway and apoptosis markers. Immunoprecipitation assay and transfection were used to determine the expression levels of proteins interacting with the pathways influenced by JI017 in prostate cancer cells. The anticancer effects induced by JI017 were evaluated. JI017 induced cell death that regulated apoptotic molecules and caused cell cycle arrest that inhibited the proliferation of cancer cells. Moreover, JI017 generated ROS. Accumulation of ROS caused ER stress through the PERK-eIF2α-CHOP and IRE1α-CHOP pathways. Furthermore, persistent activation of the UPR pathway induced by JI017 treatment triggered mitochondrial dysfunction, including dissipation of mitochondrial membrane potential, which activated intrinsic apoptotic pathway in human prostate cancer cells. The data indicated that N-acetyl-L-cysteine diminished apoptosis. We demonstrated that JI017 induced ER stress and cell death. Anticancer properties of JI017 in prostate cancer cells and in a human prostate cancer model involved ROS-mediated ER stress. Thus, JI017 treatment provides a new strategy for chemotherapy of prostate cancer.
Keywords: CHOP; JI017; ROS; cancer; er stress; mitochondria cell death.
Copyright © 2021 Kim, Ku, Hong, Kim, Kwon, Park, Jung, Shin and Ko.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
JI017 Induces Cell Autophagy and Apoptosis via Elevated Levels of Reactive Oxygen Species in Human Lung Cancer Cells.Int J Mol Sci. 2023 Apr 19;24(8):7528. doi: 10.3390/ijms24087528. Int J Mol Sci. 2023. PMID: 37108692 Free PMC article.
-
The Herbal Formula JI017 Induces ER Stress via Nox4 in Breast Cancer Cells.Antioxidants (Basel). 2021 Nov 25;10(12):1881. doi: 10.3390/antiox10121881. Antioxidants (Basel). 2021. PMID: 34942984 Free PMC article.
-
JI017, a Complex Herbal Medication, Induces Apoptosis via the Nox4-PERK-CHOP Axis in Ovarian Cancer Cells.Int J Mol Sci. 2021 Nov 12;22(22):12264. doi: 10.3390/ijms222212264. Int J Mol Sci. 2021. PMID: 34830138 Free PMC article.
-
A new strategy of promoting cisplatin chemotherapeutic efficiency by targeting endoplasmic reticulum stress.Mol Clin Oncol. 2014 Jan;2(1):3-7. doi: 10.3892/mco.2013.202. Epub 2013 Oct 18. Mol Clin Oncol. 2014. PMID: 24649299 Free PMC article. Review.
-
Oxidative stress as a catalyst in prostate cancer progression: unraveling molecular mechanisms and exploring therapeutic interventions.Discov Oncol. 2025 Apr 3;16(1):457. doi: 10.1007/s12672-025-02245-4. Discov Oncol. 2025. PMID: 40178629 Free PMC article. Review.
Cited by
-
The endoplasmic reticulum stress response in prostate cancer.Nat Rev Urol. 2022 Dec;19(12):708-726. doi: 10.1038/s41585-022-00649-3. Epub 2022 Sep 27. Nat Rev Urol. 2022. PMID: 36168057 Review.
-
Exploring the dual role of endoplasmic reticulum stress in urological cancers: Implications for tumor progression and cell death interactions.J Cell Commun Signal. 2024 Nov 3;18(4):e12054. doi: 10.1002/ccs3.12054. eCollection 2024 Dec. J Cell Commun Signal. 2024. PMID: 39691874 Free PMC article. Review.
-
JI017 Induces Cell Autophagy and Apoptosis via Elevated Levels of Reactive Oxygen Species in Human Lung Cancer Cells.Int J Mol Sci. 2023 Apr 19;24(8):7528. doi: 10.3390/ijms24087528. Int J Mol Sci. 2023. PMID: 37108692 Free PMC article.
-
The correlation between mitochondria-associated endoplasmic reticulum membranes (MAMs) and Ca2+ transport in the pathogenesis of diseases.Acta Pharmacol Sin. 2025 Feb;46(2):271-291. doi: 10.1038/s41401-024-01359-9. Epub 2024 Aug 8. Acta Pharmacol Sin. 2025. PMID: 39117969 Review.
-
Mitochondria-associated endoplasmic reticulum membrane: Overview and inextricable link with cancer.J Cell Mol Med. 2023 Apr;27(7):906-919. doi: 10.1111/jcmm.17696. Epub 2023 Feb 27. J Cell Mol Med. 2023. PMID: 36852470 Free PMC article. Review.
References
-
- Bogdanos J., Karamanolakis D., Tenta R., Tsintavis A., Milathianakis C., Mitsiades C., et al. (2003). Endocrine/paracrine/autocrine Survival Factor Activity of Bone Microenvironment Participates in the Development of Androgen Ablation and Chemotherapy Refractoriness of Prostate Cancer Metastasis in Skeleton. Endocr. Relat. Cancer 10, 279–289. 10.1677/erc.0.0100279 - DOI - PubMed
-
- Burns C. P., Spector A. A. (1994). Biochemical Effects of Lipids on Cancer Therapy. J. Nutr. Biochem. 5, 114–123. 10.1016/0955-2863(94)90082-5 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

