The Angiotensin II Type 1 Receptor-Associated Protein Attenuates Angiotensin II-Mediated Inhibition of the Renal Outer Medullary Potassium Channel in Collecting Duct Cells
- PMID: 34054566
- PMCID: PMC8160308
- DOI: 10.3389/fphys.2021.642409
The Angiotensin II Type 1 Receptor-Associated Protein Attenuates Angiotensin II-Mediated Inhibition of the Renal Outer Medullary Potassium Channel in Collecting Duct Cells
Abstract
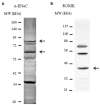
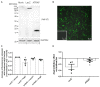
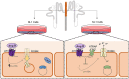
Adjustments in renal K+ excretion constitute a central mechanism for K+ homeostasis. The renal outer medullary potassium (ROMK) channel accounts for the major K+ secretory route in collecting ducts during basal conditions. Activation of the angiotensin II (Ang II) type 1 receptor (AT1R) by Ang II is known to inhibit ROMK activity under the setting of K+ dietary restriction, underscoring the role of the AT1R in K+ conservation. The present study aimed to investigate whether an AT1R binding partner, the AT1R-associated protein (ATRAP), impacts Ang II-mediated ROMK regulation in collecting duct cells and, if so, to gain insight into the potential underlying mechanisms. To this end, we overexpressed either ATRAP or β-galactosidase (LacZ; used as a control), in M-1 cells, a model line of cortical collecting duct cells. We then assessed ROMK channel activity by employing a novel fluorescence-based microplate assay. Experiments were performed in the presence of 10-10 M Ang II or vehicle for 40 min. We observed that Ang II-induced a significant inhibition of ROMK in LacZ, but not in ATRAP-overexpressed M-1 cells. Inhibition of ROMK-mediated K+ secretion by Ang II was accompanied by lower ROMK cell surface expression. Conversely, Ang II did not affect the ROMK-cell surface abundance in M-1 cells transfected with ATRAP. Additionally, diminished response to Ang II in M-1 cells overexpressing ATRAP was accompanied by decreased c-Src phosphorylation at the tyrosine 416. Unexpectedly, reduced phospho-c-Src levels were also found in M-1 cells, overexpressing ATRAP treated with vehicle, suggesting that ATRAP can also downregulate this kinase independently of Ang II-AT1R activation. Collectively, our data support that ATRAP attenuates inhibition of ROMK by Ang II in collecting duct cells, presumably by reducing c-Src activation and blocking ROMK internalization. The potential role of ATRAP in K+ homeostasis and/or disorders awaits further investigation.
Keywords: K+ channels; K+ homeostasis; angiotensin II type 1 receptor; angiotensin II type 1 receptor-associated protein; c-Src; kidney.
Copyright © 2021 Polidoro, Rebouças and Girardi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Angiotensin II type 2 receptor regulates ROMK-like K⁺ channel activity in the renal cortical collecting duct during high dietary K⁺ adaptation.Am J Physiol Renal Physiol. 2014 Oct 1;307(7):F833-43. doi: 10.1152/ajprenal.00141.2014. Epub 2014 Aug 6. Am J Physiol Renal Physiol. 2014. PMID: 25100281 Free PMC article.
-
Intrarenal suppression of angiotensin II type 1 receptor binding molecule in angiotensin II-infused mice.Am J Physiol Renal Physiol. 2010 Nov;299(5):F991-F1003. doi: 10.1152/ajprenal.00738.2009. Epub 2010 Aug 25. Am J Physiol Renal Physiol. 2010. PMID: 20739392
-
Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction.J Biol Chem. 2007 Mar 2;282(9):6455-62. doi: 10.1074/jbc.M607477200. Epub 2006 Dec 28. J Biol Chem. 2007. PMID: 17194699 Free PMC article.
-
Angiotensin II Type 1 Receptor Binding Molecule ATRAP as a Possible Modulator of Renal Sodium Handling and Blood Pressure in Pathophysiology.Curr Med Chem. 2015;22(28):3210-6. doi: 10.2174/0929867322666150821095036. Curr Med Chem. 2015. PMID: 26295465 Review.
-
The pathophysiological role of angiotensin receptor-binding protein in hypertension and kidney diseases: Oshima Award Address 2019.Clin Exp Nephrol. 2020 Apr;24(4):289-294. doi: 10.1007/s10157-020-01861-4. Epub 2020 Feb 29. Clin Exp Nephrol. 2020. PMID: 32112267 Free PMC article. Review.
Cited by
-
Exploring new horizons: angiotensin II, angiotensin II type 1 receptor, and renal outer medullary potassium channel interaction in distal convoluted tubule.Kidney Res Clin Pract. 2025 May;44(3):461-480. doi: 10.23876/j.krcp.24.023. Epub 2024 Sep 19. Kidney Res Clin Pract. 2025. PMID: 39384346 Free PMC article.
-
A Possible Link between Cell Plasticity and Renin Expression in the Collecting Duct: A Narrative Review.Int J Mol Sci. 2024 Sep 3;25(17):9549. doi: 10.3390/ijms25179549. Int J Mol Sci. 2024. PMID: 39273497 Free PMC article. Review.
References
-
- Barro-Soria R., Stindl J., Müller C., Foeckler R., Todorov V., Castrop H., et al. . (2012). Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS One 7:e49624. 10.1371/journal.pone.0049624, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

