Traumatic Brain Injury Exposure Lowers Age of Cognitive Decline in AD and Non-AD Conditions
- PMID: 34054681
- PMCID: PMC8153372
- DOI: 10.3389/fneur.2021.573401
Traumatic Brain Injury Exposure Lowers Age of Cognitive Decline in AD and Non-AD Conditions
Abstract
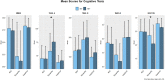
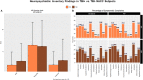
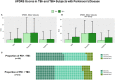
We aimed to detect the possible accelerating role of previous traumatic brain injury (TBI) exposures on the onset of later cognitive decline assessed across different brain diseases. We analyzed data from the National Alzheimer's Coordinating Center (NACC), which provide information on history of TBI and longitudinal data on cognitive and non-cognitive domains for each available subject. At the time of this investigation, a total of 609 NACC subjects resulted to have a documented history of TBI. We compared subjects with and without a history of previous TBI (of any type) at the time of their first cognitive decline assessment, and termed them, respectively, TBI+ and TBI- subjects. Three hundred and sixty-one TBI+ subjects (229 male/132 female) and 248 TBI- subjects (156 male/92 female) were available. The analyses included TBI+ and TBI- subjects with a clinical diagnosis of Mild Cognitive Impairment, Alzheimer's disease, Dementia with Lewy bodies, Progressive supranuclear palsy, Corticobasal degeneration, Frontotemporal dementia, Vascular dementia, non-AD Impairment, and Parkinson's disease. The data showed that the mean age of TBI+ subjects was lower than TBI- subjects at the time of their first cognitive decline assessment (71.6 ± 11.2 vs. 74.8 ± 9.5 year; p < 0.001). Moreover, the earlier onset of cognitive decline in TBI+ vs. TBI- subjects was independent of sex, race, attained education, APOE genotype, and importantly, clinical diagnoses. As for specific cognitive aspects, MMSE, Trail Making Test part B and WAIS-R scores did not differ between TBI+ and TBI- subjects, whereas Trail Making Test part A (p = 0.013) and Boston Naming test (p = 0.008) did. In addition, data showed that neuropsychiatric symptoms [based on Neuropsychiatry Inventory (NPI)] were much more frequent in TBI+ vs. TBI- subjects, including AD and non-AD neurodegenerative conditions such as PD. These cross-sectional analyses outcomes from longitudinally-assessed cohorts of TBI+ subjects that is, subjects with TBI exposure before the onset of cognitive decline in the contest of different neurodegenerative disorders and associated pathogenetic mechanisms, are novel, and indicate that a previous TBI exposure may act as a significant "age-lowering" factor on the onset of cognitive decline in either AD and non-AD conditions independently of demographic factors, education, APOE genotype, and current or upcoming clinical conditions.
Keywords: APOE genotype; TBI; cognitive decline; earlier-onset; neurodegenerative disorders.
Copyright © 2021 Iacono, Raiciulescu, Olsen and Perl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. (2008) 23:394–400. 10.1097/01.HTR.0000341435.52004.ac - DOI - PubMed
Grants and funding
- P50 AG005142/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P50 AG047266/AG/NIA NIH HHS/United States
- P30 AG008017/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG053760/AG/NIA NIH HHS/United States
- P30 AG062428/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P50 AG047270/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P50 AG016573/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- U54 NS115322/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

