GRK Inhibition Potentiates Glucagon-Like Peptide-1 Action
- PMID: 34054727
- PMCID: PMC8160450
- DOI: 10.3389/fendo.2021.652628
GRK Inhibition Potentiates Glucagon-Like Peptide-1 Action
Abstract
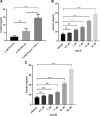
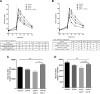
The glucagon-like peptide-1 receptor (GLP-1R) is a G-protein-coupled receptor (GPCR) whose activation results in suppression of food intake and improvement of glucose metabolism. Several receptor interacting proteins regulate the signaling of GLP-1R such as G protein-coupled receptor kinases (GRK) and β-arrestins. Here we evaluated the physiological and pharmacological impact of GRK inhibition on GLP-1R activity leveraging small molecule inhibitors of GRK2 and GRK3. We demonstrated that inhibition of GRK: i) inhibited GLP-1-mediated β-arrestin recruitment, ii) enhanced GLP-1-induced insulin secretion in isolated islets and iii) has additive effect with dipeptidyl peptidase 4 in mediating suppression of glucose excursion in mice. These findings highlight the importance of GRK to modulate GLP-1R function in vitro and in vivo. GRK inhibition is a potential therapeutic approach to enhance endogenous and pharmacologically stimulated GLP-1R signaling.
Keywords: CKD - chronic kidney disease; GLP-1; GRK2 = G protein–coupled receptor kinase 2; NASH; diabetes; insulin secretion; obesity.
Copyright © 2021 Lee, Qi, Xu, Rankin, Littrell, Xu, Bakaj and Pocai.
Conflict of interest statement
The authors are employees of Janssen.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

