The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis
- PMID: 34054735
- PMCID: PMC8150001
- DOI: 10.3389/fendo.2021.675385
The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis
Abstract
In recent decades, the mechanism underlying bone metabolic disorders based on energy metabolism has been heavily researched. Bone resorption by osteoclasts plays an important role in the occurrence and development of osteoporosis. However, the mechanism underlying the osteoclast energy metabolism disorder that interferes with bone homeostasis has not been determined. Bone resorption by osteoclasts is a process that consumes large amounts of adenosine triphosphate (ATP) produced by glycolysis and oxidative phosphorylation. In addition to glucose, fatty acids and amino acids can also be used as substrates to produce energy through oxidative phosphorylation. In this review, we summarize and analyze the energy-based phenotypic changes, epigenetic regulation, and coupling with systemic energy metabolism of osteoclasts during the development and progression of osteoporosis. At the same time, we propose a hypothesis, the compensatory recovery mechanism (involving the balance between osteoclast survival and functional activation), which may provide a new approach for the treatment of osteoporosis.
Keywords: bone homeostasis; bone resorption; energy metabolism; osteoclasts; osteoporosis.
Copyright © 2021 Da, Tao and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
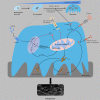
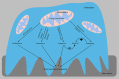

References
-
- Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. . The National Osteoporosis Foundation’s Position Statement on Peak Bone Mass Development and Lifestyle Factors: A Systematic Review and Implementation Recommendations. Osteoporosis Int (2016) 27:1281–386. 10.1007/s00198-015-3440-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

