Therapeutic Potential of Exploiting Autophagy Cascade Against Coronavirus Infection
- PMID: 34054782
- PMCID: PMC8160449
- DOI: 10.3389/fmicb.2021.675419
Therapeutic Potential of Exploiting Autophagy Cascade Against Coronavirus Infection
Abstract
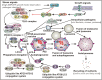
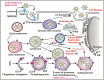
Since its emergence in December 2019 in Wuhan, China, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) created a worldwide pandemic of coronavirus disease (COVID-19) with nearly 136 million cases and approximately 3 million deaths. Recent studies indicate that like other coronaviruses, SARS-CoV-2 also hijacks or usurps various host cell machineries including autophagy for its replication and disease pathogenesis. Double membrane vesicles generated during initiation of autophagy cascade act as a scaffold for the assembly of viral replication complexes and facilitate RNA synthesis. The use of autophagy inhibitors - chloroquine and hydroxychloroquine initially appeared to be as a potential treatment strategy of COVID-19 patients but later remained at the center of debate due to high cytotoxic effects. In the absence of a specific drug or vaccine, there is an urgent need for a safe, potent as well as affordable drug to control the disease spread. Given the intricate connection between autophagy machinery and viral pathogenesis, the question arises whether targeting autophagy pathway might show a path to fight against SARS-CoV-2 infection. In this review we will discuss about our current knowledge linking autophagy to coronaviruses and how that is being utilized to repurpose autophagy modulators as potential COVID-19 treatment.
Keywords: COVID-19; SARS-CoV-2; autophagy; coronaviruses (CoVs); virophagy.
Copyright © 2021 Maity and Saha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Autophagy/virophagy: a "disposal strategy" to combat COVID-19.Autophagy. 2020 Dec;16(12):2271-2272. doi: 10.1080/15548627.2020.1782022. Epub 2020 Jun 24. Autophagy. 2020. PMID: 32578486 Free PMC article.
-
Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19.Int J Biol Sci. 2020 Mar 15;16(10):1724-1731. doi: 10.7150/ijbs.45498. eCollection 2020. Int J Biol Sci. 2020. PMID: 32226290 Free PMC article. Review.
-
The role of autophagy in controlling SARS-CoV-2 infection: An overview on virophagy-mediated molecular drug targets.Cell Biol Int. 2021 Aug;45(8):1599-1612. doi: 10.1002/cbin.11609. Epub 2021 Apr 23. Cell Biol Int. 2021. PMID: 33818861 Free PMC article. Review.
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status.J Infect Public Health. 2020 Nov;13(11):1601-1610. doi: 10.1016/j.jiph.2020.07.011. Epub 2020 Aug 4. J Infect Public Health. 2020. PMID: 32778421 Free PMC article.
Cited by
-
Parkinson's Disease and SARS-CoV-2 Infection: Particularities of Molecular and Cellular Mechanisms Regarding Pathogenesis and Treatment.Biomedicines. 2022 Apr 26;10(5):1000. doi: 10.3390/biomedicines10051000. Biomedicines. 2022. PMID: 35625737 Free PMC article. Review.
-
Autophagy and Respiratory Viruses: Mechanisms, Viral Exploitation, and Therapeutic Insights.Cells. 2025 Mar 12;14(6):418. doi: 10.3390/cells14060418. Cells. 2025. PMID: 40136667 Free PMC article. Review.
-
COVID-19 and retinal degenerative diseases: Promising link "Kaempferol".Curr Opin Pharmacol. 2022 Jun;64:102231. doi: 10.1016/j.coph.2022.102231. Epub 2022 Apr 14. Curr Opin Pharmacol. 2022. PMID: 35544976 Free PMC article. Review.
-
Temporal proteomic analyses of human lung cells distinguish high pathogenicity influenza viruses and coronaviruses from low pathogenicity viruses.Front Microbiol. 2022 Oct 10;13:994512. doi: 10.3389/fmicb.2022.994512. eCollection 2022. Front Microbiol. 2022. PMID: 36299731 Free PMC article.
-
Coronavirus Usurps the Autophagy-Lysosome Pathway and Induces Membranes Rearrangement for Infection and Pathogenesis.Front Microbiol. 2022 Mar 2;13:846543. doi: 10.3389/fmicb.2022.846543. eCollection 2022. Front Microbiol. 2022. PMID: 35308399 Free PMC article. Review.
References
-
- Albert S., Serova M., Dreyer C., Sablin M. P., Faivre S., Raymond E. (2010). New inhibitors of the mammalian target of rapamycin signaling pathway for cancer. Expert. Opin. Invest. Drugs 19 919–930. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

