Latent TGF-β Activation Is a Hallmark of the Tenascin Family
- PMID: 34054795
- PMCID: PMC8155481
- DOI: 10.3389/fimmu.2021.613438
Latent TGF-β Activation Is a Hallmark of the Tenascin Family
Abstract
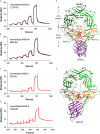
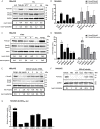
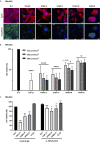
Transforming growth factor-β (TGF-β) isoforms are secreted as inactive complexes formed through non-covalent interactions between bioactive TGF-β entities and their N-terminal pro-domains called latency-associated peptides (LAP). Extracellular activation of latent TGF-β within this complex is a crucial step in the regulation of TGF-β activity for tissue homeostasis and immune cell function. We previously showed that the matrix glycoprotein Tenascin-X (TN-X) interacted with the small latent TGF-β complex and triggered the activation of the latent cytokine into a bioactive TGF-β. This activation most likely occurs through a conformational change within the latent TGF-β complex and requires the C-terminal fibrinogen-like (FBG) domain of the glycoprotein. As the FBG-like domain is highly conserved among the Tenascin family members, we hypothesized that Tenascin-C (TN-C), Tenascin-R (TN-R) and Tenascin-W (TN-W) might share with TN-X the ability to regulate TGF-β bioavailability through their C-terminal domain. Here, we demonstrate that purified recombinant full-length Tenascins associate with the small latent TGF-β complex through their FBG-like domains. This association promotes activation of the latent cytokine and subsequent TGF-β cell responses in mammary epithelial cells, such as cytostasis and epithelial-to-mesenchymal transition (EMT). Considering the pleiotropic role of TGF-β in numerous physiological and pathological contexts, our data indicate a novel common function for the Tenascin family in the regulation of tissue homeostasis under healthy and pathological conditions.
Keywords: immune cell modulation; latent TGF-β activation; tenascins; tissue homeostasis; transforming growth factor (TGF)-β; tumor microenvironment.
Copyright © 2021 Aubert, Mercier-Gouy, Aguero, Berthier, Liot, Prigent, Alcaraz, Verrier, Terreux, Moali, Lambert and Valcourt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

