Intra-Abdominal Lipopolysaccharide Clearance and Inactivation in Peritonitis: Key Roles for Lipoproteins and the Phospholipid Transfer Protein
- PMID: 34054798
- PMCID: PMC8149805
- DOI: 10.3389/fimmu.2021.622935
Intra-Abdominal Lipopolysaccharide Clearance and Inactivation in Peritonitis: Key Roles for Lipoproteins and the Phospholipid Transfer Protein
Abstract
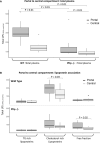
Introduction: During peritonitis, lipopolysaccharides (LPS) cross the peritoneum and pass through the liver before reaching the central compartment. The aim of the present study was to investigate the role of lipoproteins and phospholipid transfer protein (PLTP) in the early stages of LPS detoxification.
Material and methods: Peritonitis was induced by intra-peritoneal injection of LPS in mice. We analyzed peritoneal fluid, portal and central blood. Lipoprotein fractions were obtained by ultracentrifugation and fast protein liquid chromatography. LPS concentration and activity were measured by liquid chromatography coupled with mass spectrometry and limulus amoebocyte lysate. Wild-type mice were compared to mice knocked out for PLTP.
Results: In mice expressing PLTP, LPS was able to bind to HDL in the peritoneal compartment, and this was maintained in plasma from portal and central blood. A hepatic first-pass effect of HDL-bound LPS was observed in wild-type mice. LPS binding to HDL resulted in an early arrival of inactive LPS in the central blood of wild-type mice.
Conclusion: PLTP promotes LPS peritoneal clearance and neutralization in a model of peritonitis. This mechanism involves the early binding of LPS to lipoproteins inside the peritoneal cavity, which promotes LPS translocation through the peritoneum and its uptake by the liver.
Keywords: endotoxemia; inflammation; lipopolysaccharides 4; lipoproteins; peritonitis; phospholipid transfer protein; sepsis.
Copyright © 2021 Nguyen, Pallot, Jalil, Tavernier, Dusuel, Le Guern, Lagrost, Pais de Barros, Choubley, Bergas, Guinot, Masson, Bouhemad and Gautier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

