Postpartum HPV Vaccination Rate and Differences in Background Characteristics Between HPV Vaccinated and Unvaccinated Postpartum Women: Strict Monitoring and Follow-Up of Postpartum HPV Vaccination Program
- PMID: 34054800
- PMCID: PMC8149888
- DOI: 10.3389/fimmu.2021.626582
Postpartum HPV Vaccination Rate and Differences in Background Characteristics Between HPV Vaccinated and Unvaccinated Postpartum Women: Strict Monitoring and Follow-Up of Postpartum HPV Vaccination Program
Abstract
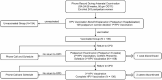
There is a need to increase the vaccine completion rates in women who have already received human papillomavirus (HPV) vaccines. With vaccines requiring multiple doses, designing a vaccination control program and increasing the proportion of women who complete vaccination are critical and remain as huge challenges. Currently, there are no published reports on the differences in the background characteristics between postpartum women who are vaccinated or unvaccinated against HPV. This study aimed to determine the vaccination rates of the second and third doses of HPV vaccination utilizing an achievable HPV vaccination program in postpartum women. In this retrospective study, 243 postpartum women attending Chiayi Chang Gung Memorial Hospital between March and September 2014 were enrolled. These women were classified into two groups: one group received the HPV vaccine under a practical, controlled postpartum HPV vaccination program, and the other group did not. The rates for the second and third rounds of HPV vaccination in postpartum women were calculated. The differences in the background characteristics between the two groups were determined using the Student's t test, chi-square test or Fisher's exact test, and the multiple logistic models, as appropriate. Under the controlled postpartum HPV vaccination program, the completion rate for the three doses of postpartum HPV vaccination was 97.2%. Significant differences were observed according to maternal age, gender of the newborn, and postpartum Pap smear results between the two groups in our study. In conclusion, the controlled postpartum HPV vaccination program is a reasonable method for achieving an excellent completion rate for the three doses of postpartum HPV vaccination and may be a good model for any multiple-dose vaccination protocol.
Keywords: human papillomavirus (HPV); papillomavirus vaccines; postpartum period; uterine cervical neoplasms; vaccination.
Copyright © 2021 Lee, Tseng, Chang, Lee and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Chen J, Gopala K, Puthatta A, Struyf F, Rosillon D. Prevalence and Incidence of Human Papillomavirus (HPV) Infection Before and After Pregnancy: Pooled Analysis of the Control Arms of Efficacy Trials of HPV-16/18 AS04-Adjuvanted Vaccine. Open Forum Infect Dis (2019) 6:ofz486. 10.1093/ofid/ofz486 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials