Differences in Maturation Status and Immune Phenotypes of Circulating Helios+ and Helios- Tregs and Their Disrupted Correlations With Monocyte Subsets in Autoantibody-Positive T1D Individuals
- PMID: 34054801
- PMCID: PMC8149963
- DOI: 10.3389/fimmu.2021.628504
Differences in Maturation Status and Immune Phenotypes of Circulating Helios+ and Helios- Tregs and Their Disrupted Correlations With Monocyte Subsets in Autoantibody-Positive T1D Individuals
Abstract
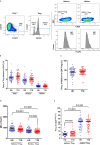
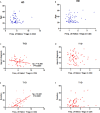
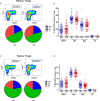
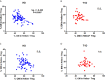
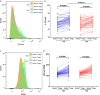
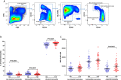
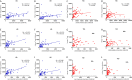
CD4 Tregs are involved in the regulation of various autoimmune diseases but believed to be highly heterogeneous. Studies have indicated that Helios controls a distinct subset of functional Tregs. However, the immunological changes in circulating Helios+ and Helios- Tregs are not fully explored in type 1 diabetes (T1D). Here, we elucidated the differences in maturation status and immune regulatory phenotypes of Helios+ and Helios- Tregs and their correlations with monocyte subsets in T1D individuals. As CD25-/low FOXP3+ Tregs also represent a subset of functional Tregs, we defined Tregs as FOXP3+CD127-/low and examined circulating Helios+ and Helios- Treg subpopulations in 68 autoantibody-positive T1D individuals and 68 age-matched healthy controls. We found that expression of both FOXP3 and CTLA4 diminished in Helios- Tregs, while the proportion of CD25-/low Tregs increased in Helios+ Tregs of T1D individuals. Although the frequencies of neither Helios+ nor Helios- Tregs were affected by investigated T1D genetic risk loci, Helios+ Tregs correlated with age at T1D diagnosis negatively and disease duration positively. Moreover, the negative correlation between central and effector memory proportions of Helios+ Tregs in healthy controls was disrupted in T1D individuals. Finally, regulatory non-classical and intermediate monocytes also decreased in T1D individuals, and positive correlations between these regulatory monocytes and Helios+/Helios- Treg subsets in healthy controls disappeared in T1D individuals. In conclusion, we demonstrated the alternations in maturation status and immune phenotypes in Helios+ and Helios- Treg subsets and revealed the missing association between these Treg subsets and monocyte subsets in T1D individuals, which might point out another option for elucidating T1D mechanisms.
Keywords: Helios; Tregs; monocytes; regulatory; type 1 diabetes.
Copyright © 2021 Zhang, Zhang, Shi, Shen, Lv, Chen, Feng, Chen, Xu, Yang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

