Rapamycin Modulates the Proinflammatory Memory-Like Response of Microglia Induced by BAFF
- PMID: 34054807
- PMCID: PMC8158300
- DOI: 10.3389/fimmu.2021.639049
Rapamycin Modulates the Proinflammatory Memory-Like Response of Microglia Induced by BAFF
Abstract
Background: Recently trained immunity of microglia provided an opportunity to study the chronic effect of microglial activation and its metabolic rewiring in neuroimmunological diseases. Since elevated levels of B cell-activating factor (BAFF) have been proved to be associated with some chronic neuroimmunological disorders. Here, we used the trained innate immunity model to analyze the effect of BAFF, a vital regulator of the adaptive immune system, on long-term microglial activation and metabolic reprogramming in vitro and in vivo.
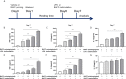
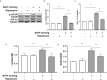
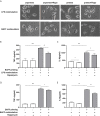
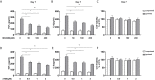
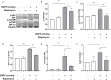
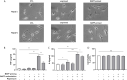
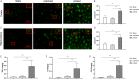
Methods and results: In vitro, BV2 cells and mouse primary microglial cells were incubated with BAFF for 24 h (BAFF priming). After 5 days of resting, microglia were restimulated with LPS (LPS restimulation) or BAFF (BAFF restimulation). BAFF priming induced a pro-inflammatory trained immunity-phenotype of both BV2 cells and primary microglial cells, which was indicated by morphological change, secretion of pro-inflammatory cytokine and chemokine upon LPS restimulation or BAFF restimulation. The production of lactate and NAD+/NADH ratio were elevated 5 days after BAFF priming. The activation of the Akt/mTOR/HIF-1α pathway was induced by BAFF priming and lasted for 5 days. Pretreating the BV2 cells or mouse primary microglial cells with rapamycin blocked mTOR/HIF-1α activation and cellular metabolic reprogramming induced by BAFF training. Consistently, rapamycin efficiently suppressed the trained immunity-like responses of microglia triggered by BAFF. In vivo, adult male mice were treated with BAFF by intracerebroventricular injection for priming and 7 days later with BAFF for restimulation. BAFF training activated microglia in the cortex and hippocampus. The production of proinflammatory cytokines and chemokines was elevated after BAFF training.
Conclusion: Our current data, for the first time, demonstrate that BAFF priming induces a proinflammatory memory-like response of microglia not only to LPS but also to BAFF itself. Rapamycin inhibits microglial priming triggered by BAFF through targeting the mTOR/HIF-1α signaling pathway. Our data reveal a novel role of BAFF in trained immunity and that rapamycin may be a potential therapeutic target of neuroimmunological diseases.
Keywords: B cell-activating factor; aerobic glycolysis; mammalian target of rapamycin; microglia; trained immunity.
Copyright © 2021 Wang, Yang, Hou, Xu, Yun, Qin and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Modelling Microglial Innate Immune Memory In Vitro: Understanding the Role of Aerobic Glycolysis in Innate Immune Memory.Int J Mol Sci. 2023 May 18;24(10):8967. doi: 10.3390/ijms24108967. Int J Mol Sci. 2023. PMID: 37240311 Free PMC article.
-
Induction of Innate Immune Memory in LPS-Primed Microglial Cells by Water-Soluble Chitosan.Biomed Res Int. 2024 Nov 30;2024:8027006. doi: 10.1155/bmri/8027006. eCollection 2024. Biomed Res Int. 2024. PMID: 39654846 Free PMC article.
-
Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation.J Neuroinflammation. 2020 Jan 11;17(1):18. doi: 10.1186/s12974-019-1644-8. J Neuroinflammation. 2020. PMID: 31926553 Free PMC article.
-
Priming Microglia for Innate Immune Memory in the Brain.Trends Immunol. 2019 Apr;40(4):358-374. doi: 10.1016/j.it.2019.02.001. Epub 2019 Mar 1. Trends Immunol. 2019. PMID: 30833177 Review.
-
Review: microglia of the aged brain: primed to be activated and resistant to regulation.Neuropathol Appl Neurobiol. 2013 Feb;39(1):19-34. doi: 10.1111/j.1365-2990.2012.01306.x. Neuropathol Appl Neurobiol. 2013. PMID: 23039106 Free PMC article. Review.
Cited by
-
Trained immunity: A Yin-Yang balance.MedComm (2020). 2022 Mar 6;3(1):e121. doi: 10.1002/mco2.121. eCollection 2022 Mar. MedComm (2020). 2022. PMID: 35281787 Free PMC article. Review.
-
The Triangle Relationship Between Long Noncoding RNA, RIG-I-like Receptor Signaling Pathway, and Glycolysis.Front Microbiol. 2021 Nov 30;12:807737. doi: 10.3389/fmicb.2021.807737. eCollection 2021. Front Microbiol. 2021. PMID: 34917069 Free PMC article. Review.
-
Modelling Microglial Innate Immune Memory In Vitro: Understanding the Role of Aerobic Glycolysis in Innate Immune Memory.Int J Mol Sci. 2023 May 18;24(10):8967. doi: 10.3390/ijms24108967. Int J Mol Sci. 2023. PMID: 37240311 Free PMC article.
-
Metabolic Reprogramming of Microglia in Sepsis-Associated Encephalopathy: Insights from Neuroinflammation.Curr Neuropharmacol. 2023;21(9):1992-2005. doi: 10.2174/1570159X21666221216162606. Curr Neuropharmacol. 2023. PMID: 36529923 Free PMC article. Review.
-
Disturbance of neuron-microglia crosstalk mediated by GRP78 in Neuropsychiatric systemic lupus erythematosus mice.J Neuroinflammation. 2023 Jun 26;20(1):150. doi: 10.1186/s12974-023-02832-8. J Neuroinflammation. 2023. PMID: 37365565 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

