Immune Landscape of Gastric Carcinoma Tumor Microenvironment Identifies a Peritoneal Relapse Relevant Immune Signature
- PMID: 34054812
- PMCID: PMC8155484
- DOI: 10.3389/fimmu.2021.651033
Immune Landscape of Gastric Carcinoma Tumor Microenvironment Identifies a Peritoneal Relapse Relevant Immune Signature
Abstract
Background: Gastric cancer (GC) still represents the third leading cause of cancer-related death worldwide. Peritoneal relapse (PR) is the most frequent metastasis occurring among patients with advanced gastric cancer. Increasingly more evidence have clarified the tumor immune microenvironment (TIME) may predict survival and have clinical significance in GC. However, tumor-transcriptomics based immune signatures derived from immune profiling have not been established for predicting the peritoneal recurrence of the advanced GC.
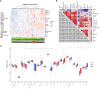
Methods: In this study, we depict the immune landscape of GC by using transcriptome profiling and clinical characteristics retrieved from GSE62254 of Gene Expression Omnibus (GEO). Immune cell infiltration score was evaluated via single-sample gene set enrichment (ssGSEA) analysis algorithm. The least absolute shrinkage and selection operator (LASSO) Cox regression algorithm was used to select the valuable immune cells and construct the final model for the prediction of PR. The receiver operating characteristic (ROC) curve and the Kaplan-Meier curve were used to check the accuracy of PRIs. Gene Set Enrichment Analysis (GSEA) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed to explore the molecular pathways associated with PRIs.
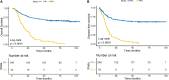
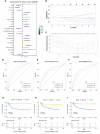
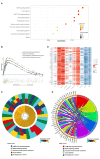
Results: A peritoneal recurrence related immune score (PRIs) with 10 immune cells was constructed. Compared to the low-PRIs group, the high-PRIs group had a greater risk. The upregulation of the focal adhesion signaling was observed in the high-PRIs subtype by GSEA and KEGG. Multivariate analysis found that both in the internal training cohort and the internal validation cohort, PRIs was a stable and independent predictor for PR. A nomogram that integrated clinicopathological features and PRIs to predict peritoneal relapse was constructed. Subgroup analysis indicated that the PRIs could obviously distinguish peritoneal recurrence in different molecular subtypes, pathological stages and Lauren subtypes, in which PRIs of Epithelial-Mesenchymal Transitions (EMT) subtype, III-IV stage and diffuse subtype are higher respectively.
Conclusion: Overall, we performed a comprehensive evaluation of the immune landscape of GC and constructed a predictive PR model based on the immune cell infiltration. The PRIs represents novel promising feature of predicting peritoneal recurrence of GC and sheds light on the improvement of the personalized management of GC patients after surgery.
Keywords: LASSO; TME; gastric cancer; immune signature; peritoneal relapse.
Copyright © 2021 Zhang, Li, Yu, Li, Song, Chen, Fan, Liu and Qu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, et al. . Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (Cyto-CHIP Study): A Propensity Score Analysis. J Clin Oncol (2019) 37(23):2028–40. 10.1200/JCO.18.01688 - DOI - PubMed
-
- Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, et al. . Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Improves Survival of Patients With Peritoneal Carcinomatosis From Gastric Cancer: Final Results of a Phase III Randomized Clinical Trial. Ann Surg Oncol (2011) 18(6):1575–81. 10.1245/s10434-011-1631-5 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

