Peroxiredoxin 3 Inhibits Acetaminophen-Induced Liver Pyroptosis Through the Regulation of Mitochondrial ROS
- PMID: 34054813
- PMCID: PMC8155593
- DOI: 10.3389/fimmu.2021.652782
Peroxiredoxin 3 Inhibits Acetaminophen-Induced Liver Pyroptosis Through the Regulation of Mitochondrial ROS
Abstract
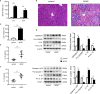
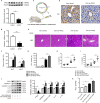
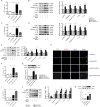
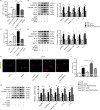
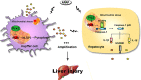
Pyroptosis is a newly discovered form of cell death. Peroxiredoxin 3 (PRX3) plays a crucial role in scavenging reactive oxygen species (ROS), but its hepatoprotective capacity in acetaminophen (APAP)-induced liver disease remains unclear. The aim of this study was to assess the role of PRX3 in the regulation of pyroptosis during APAP-mediated hepatotoxicity. We demonstrated that pyroptosis occurs in APAP-induced liver injury accompanied by intense oxidative stress and inflammation, and liver specific PRX3 silencing aggravated the initiation of pyroptosis and liver injury after APAP intervention. Notably, excessive mitochondrial ROS (mtROS) was observed to trigger pyroptosis by activating the NLRP3 inflammasome, which was ameliorated by Mito-TEMPO treatment, indicating that the anti-pyroptotic role of PRX3 relies on its powerful ability to regulate mtROS. Overall, PRX3 regulates NLRP3-dependent pyroptosis in APAP-induced liver injury by targeting mitochondrial oxidative stress.
Keywords: APAP; NLRP3 inflammasome; PRX3; mitochondrial ROS (mtROS); pyroptosis.
Copyright © 2021 Wang, Zhao, Wang, Sun, Zou, Li, Liu, Lin, Zhou, Ning, Tian and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Caveolin-1 ameliorates acetaminophen-aggravated inflammatory damage and lipid deposition in non-alcoholic fatty liver disease via the ROS/TXNIP/NLRP3 pathway.Int Immunopharmacol. 2023 Jan;114:109558. doi: 10.1016/j.intimp.2022.109558. Epub 2022 Dec 21. Int Immunopharmacol. 2023. PMID: 36700765
-
Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells.Biofactors. 2018 Mar;44(2):123-136. doi: 10.1002/biof.1395. Epub 2017 Nov 29. Biofactors. 2018. PMID: 29193391
-
Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis.Nanoscale. 2018 May 17;10(19):9141-9152. doi: 10.1039/c8nr00554k. Nanoscale. 2018. PMID: 29722780
-
The role of mitochondria in NLRP3 inflammasome activation.Mol Immunol. 2018 Nov;103:115-124. doi: 10.1016/j.molimm.2018.09.010. Epub 2018 Sep 21. Mol Immunol. 2018. PMID: 30248487 Review.
-
The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes.Int J Nanomedicine. 2019 Nov 7;14:8787-8804. doi: 10.2147/IJN.S212907. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31806972 Free PMC article. Review.
Cited by
-
Acetaminophen-induced liver injury: Molecular mechanism and treatments from natural products.Front Pharmacol. 2023 Mar 27;14:1122632. doi: 10.3389/fphar.2023.1122632. eCollection 2023. Front Pharmacol. 2023. PMID: 37050900 Free PMC article. Review.
-
Liver injury in sepsis: manifestations, mechanisms and emerging therapeutic strategies.Front Immunol. 2025 Mar 28;16:1575554. doi: 10.3389/fimmu.2025.1575554. eCollection 2025. Front Immunol. 2025. PMID: 40226624 Free PMC article. Review.
-
Acetaminophen Hepatotoxicity: Paradigm for Understanding Mechanisms of Drug-Induced Liver Injury.Annu Rev Pathol. 2024 Jan 24;19:453-478. doi: 10.1146/annurev-pathmechdis-051122-094016. Annu Rev Pathol. 2024. PMID: 38265880 Free PMC article. Review.
-
The protective effect of Thai rice bran on N-acetyl-ρ-aminophen-induced hepatotoxicity in mice.Res Pharm Sci. 2024 Apr 1;19(2):188-202. doi: 10.4103/RPS.RPS_210_23. eCollection 2024 Apr. Res Pharm Sci. 2024. PMID: 39035586 Free PMC article.
-
Effect of NLRP3 gene knockdown on pyroptosis and ferroptosis in diabetic cardiomyopathy injury.BMC Cardiovasc Disord. 2024 Jul 10;24(1):351. doi: 10.1186/s12872-024-04010-x. BMC Cardiovasc Disord. 2024. PMID: 38987672 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

