DDX3X Links NLRP11 to the Regulation of Type I Interferon Responses and NLRP3 Inflammasome Activation
- PMID: 34054816
- PMCID: PMC8158815
- DOI: 10.3389/fimmu.2021.653883
DDX3X Links NLRP11 to the Regulation of Type I Interferon Responses and NLRP3 Inflammasome Activation
Abstract
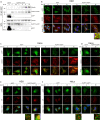
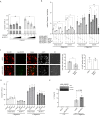
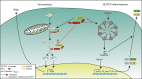
Tight regulation of inflammatory cytokine and interferon (IFN) production in innate immunity is pivotal for optimal control of pathogens and avoidance of immunopathology. The human Nod-like receptor (NLR) NLRP11 has been shown to regulate type I IFN and pro-inflammatory cytokine responses. Here, we identified the ATP-dependent RNA helicase DDX3X as a novel binding partner of NLRP11, using co-immunoprecipitation and LC-MS/MS. DDX3X is known to enhance type I IFN responses and NLRP3 inflammasome activation. We demonstrate that NLRP11 can abolish IKKϵ-mediated phosphorylation of DDX3X, resulting in lower type I IFN induction upon viral infection. These effects were dependent on the LRR domain of NLRP11 that we mapped as the interaction domain for DDX3X. In addition, NLRP11 also suppressed NLRP3-mediated caspase-1 activation in an LRR domain-dependent manner, suggesting that NLRP11 might sequester DDX3X and prevent it from promoting NLRP3-induced inflammasome activation. Taken together, our data revealed DDX3X as a central target of NLRP11, which can mediate the effects of NLRP11 on type I IFN induction as well as NLRP3 inflammasome activation. This expands our knowledge of the molecular mechanisms underlying NLRP11 function in innate immunity and suggests that both NLRP11 and DDX3X might be promising targets for modulation of innate immune responses.
Keywords: DEAD-box helicase; IL-1; anti-viral; inflammasome; innate immunity; nod-like receptors; type I interferon.
Copyright © 2021 Kienes, Bauer, Gottschild, Mirza, Pfannstiel, Schröder and Kufer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
DDX3X coordinates host defense against influenza virus by activating the NLRP3 inflammasome and type I interferon response.J Biol Chem. 2021 Jan-Jun;296:100579. doi: 10.1016/j.jbc.2021.100579. Epub 2021 Mar 23. J Biol Chem. 2021. PMID: 33766561 Free PMC article.
-
The NLR family pyrin domain-containing 11 protein contributes to the regulation of inflammatory signaling.J Biol Chem. 2018 Feb 23;293(8):2701-2710. doi: 10.1074/jbc.RA117.000152. Epub 2018 Jan 4. J Biol Chem. 2018. PMID: 29301940 Free PMC article.
-
TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury.Clin Transl Med. 2022 Jun;12(6):e894. doi: 10.1002/ctm2.894. Clin Transl Med. 2022. PMID: 35692100 Free PMC article.
-
Advances in the molecular mechanisms of NLRP3 inflammasome activators and inactivators.Biochem Pharmacol. 2020 May;175:113863. doi: 10.1016/j.bcp.2020.113863. Epub 2020 Feb 17. Biochem Pharmacol. 2020. PMID: 32081791 Review.
-
The multifaceted roles of NLRP3-modulating proteins in virus infection.Front Immunol. 2022 Aug 30;13:987453. doi: 10.3389/fimmu.2022.987453. eCollection 2022. Front Immunol. 2022. PMID: 36110852 Free PMC article. Review.
Cited by
-
The human DEAD-box helicase DDX3X as a regulator of mRNA translation.Front Cell Dev Biol. 2022 Oct 25;10:1033684. doi: 10.3389/fcell.2022.1033684. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36393867 Free PMC article. Review.
-
Angiogenin-mediated tsRNAs control inflammation and metabolic disorder by regulating NLRP3 inflammasome.Cell Death Differ. 2024 Aug;31(8):1057-1069. doi: 10.1038/s41418-024-01311-8. Epub 2024 May 13. Cell Death Differ. 2024. PMID: 38740959 Free PMC article.
-
Proliferating Microglia Exhibit Unique Transcriptional and Functional Alterations in Alzheimer's Disease.ASN Neuro. 2025;17(1):2506406. doi: 10.1080/17590914.2025.2506406. Epub 2025 May 19. ASN Neuro. 2025. PMID: 40387894 Free PMC article.
-
A prediction of the CRNDE role by modulating NF-κB pathway in inflammatory bowel disease (IBD).Biochem Biophys Rep. 2024 May 11;38:101731. doi: 10.1016/j.bbrep.2024.101731. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38766384 Free PMC article.
-
Decoding NLRP3 Inflammasome Activation in Alzheimer's Disease: A Focus on Receptor Dynamics.Mol Neurobiol. 2025 Aug;62(8):10792-10812. doi: 10.1007/s12035-025-04918-1. Epub 2025 Apr 15. Mol Neurobiol. 2025. PMID: 40232645 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

