Ironing Out the Details: How Iron Orchestrates Macrophage Polarization
- PMID: 34054839
- PMCID: PMC8149954
- DOI: 10.3389/fimmu.2021.669566
Ironing Out the Details: How Iron Orchestrates Macrophage Polarization
Abstract
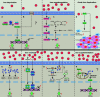
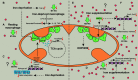
Iron fine-tunes innate immune responses, including macrophage inflammation. In this review, we summarize the current understanding about the iron in dictating macrophage polarization. Mechanistically, iron orchestrates macrophage polarization through several aspects, including cellular signaling, cellular metabolism, and epigenetic regulation. Therefore, iron modulates the development and progression of multiple macrophage-associated diseases, such as cancer, atherosclerosis, and liver diseases. Collectively, this review highlights the crucial role of iron for macrophage polarization, and indicates the potential application of iron supplementation as an adjuvant therapy in different inflammatory disorders relative to the balance of macrophage polarization.
Keywords: epigenetics; inflammation; iron; macrophage; metabolism.
Copyright © 2021 Xia, Li, Wu, Zhang, Chen, Ma and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Macrophage Polarization and Its Role in Liver Disease.Front Immunol. 2021 Dec 14;12:803037. doi: 10.3389/fimmu.2021.803037. eCollection 2021. Front Immunol. 2021. PMID: 34970275 Free PMC article. Review.
-
Epigenetic regulation of macrophages: from homeostasis maintenance to host defense.Cell Mol Immunol. 2020 Jan;17(1):36-49. doi: 10.1038/s41423-019-0315-0. Epub 2019 Oct 29. Cell Mol Immunol. 2020. PMID: 31664225 Free PMC article. Review.
-
Metabolic and epigenetic regulation of macrophage polarization in atherosclerosis: Molecular mechanisms and targeted therapies.Pharmacol Res. 2025 Feb;212:107588. doi: 10.1016/j.phrs.2025.107588. Epub 2025 Jan 6. Pharmacol Res. 2025. PMID: 39778637 Review.
-
Metabolic reprogramming of macrophages during infections and cancer.Cancer Lett. 2019 Jun 28;452:14-22. doi: 10.1016/j.canlet.2019.03.015. Epub 2019 Mar 21. Cancer Lett. 2019. PMID: 30905817 Review.
-
Macrophage polarization: the epigenetic point of view.Curr Opin Lipidol. 2014 Oct;25(5):367-73. doi: 10.1097/MOL.0000000000000109. Curr Opin Lipidol. 2014. PMID: 25188918 Review.
Cited by
-
The Effects of Vitamins and Micronutrients on Helicobacter pylori Pathogenicity, Survival, and Eradication: A Crosstalk between Micronutrients and Immune System.J Immunol Res. 2022 Mar 16;2022:4713684. doi: 10.1155/2022/4713684. eCollection 2022. J Immunol Res. 2022. PMID: 35340586 Free PMC article. Review.
-
Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis.Curr Issues Mol Biol. 2022 Dec 7;44(12):6189-6204. doi: 10.3390/cimb44120422. Curr Issues Mol Biol. 2022. PMID: 36547083 Free PMC article.
-
Glycinergic Signaling in Macrophages and Its Application in Macrophage-Associated Diseases.Front Immunol. 2021 Oct 5;12:762564. doi: 10.3389/fimmu.2021.762564. eCollection 2021. Front Immunol. 2021. PMID: 34675940 Free PMC article. Review.
-
HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease.Biomedicines. 2025 Mar 10;13(3):683. doi: 10.3390/biomedicines13030683. Biomedicines. 2025. PMID: 40149659 Free PMC article. Review.
-
Multidimensional applications of prussian blue-based nanoparticles in cancer immunotherapy.J Nanobiotechnology. 2025 Mar 3;23(1):161. doi: 10.1186/s12951-025-03236-x. J Nanobiotechnology. 2025. PMID: 40033359 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

