Rituximab Associated Hypogammaglobulinemia in Autoimmune Disease
- PMID: 34054846
- PMCID: PMC8149951
- DOI: 10.3389/fimmu.2021.671503
Rituximab Associated Hypogammaglobulinemia in Autoimmune Disease
Abstract
Objective: To evaluate the characteristics of patients with autoimmune disease with hypogammaglobulinemia following rituximab (RTX) and describe their long-term outcomes, including those who commenced immunoglobulin replacement therapy.
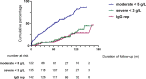
Methods: Patients received RTX for autoimmune disease between 2003 and 2012 with immunoglobulin G (IgG) <7g/L were included in this retrospective series. Hypogammaglobulinemia was classified by nadir IgG subgroups of 5 to <7g/L (mild), 3 to <5g/L (moderate) and <3g/L (severe). Characteristics of patients were compared across subgroups and examined for factors associated with greater likelihood of long term hypogammaglobulinemia or immunoglobulin replacement.
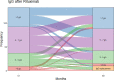
Results: 142 patients were included; 101 (71%) had anti-neutrophil cytoplasm antibody (ANCA) associated vasculitis (AAV), 18 (13%) systemic lupus erythematosus (SLE) and 23 (16%) other conditions. Mean follow-up was 97.2 months from first RTX. Hypogammaglobulinemia continued to be identified during long-term follow-up. Median time to IgG <5g/L was 22.5 months. Greater likelihood of moderate hypogammaglobulinemia (IgG <5g/L) and/or use of immunoglobulin replacement therapy at 60 months was observed in patients with prior cyclophosphamide exposure (odds ratio (OR) 3.60 [95% confidence interval (CI) 1.03 - 12.53], glucocorticoid use at 12 months [OR 7.48 (95% CI 1.28 - 43.55], lower nadir IgG within 12 months of RTX commencement [OR 0.68 (95% CI 0.51 - 0.90)] and female sex [OR 8.57 (95% CI 2.07 - 35.43)]. Immunoglobulin replacement was commenced in 29/142 (20%) and associated with reduction in infection rates, but not severe infection rates.
Conclusion: Hypogammaglobulinemia continues to occur in long-term follow-up post-RTX. In patients with recurrent infections, immunoglobulin replacement reduced rates of non-severe infections.
Keywords: B-cell; autoimmune disease; hypogammaglobulinemia; immunoglobulin replacement therapy; rituximab.
Copyright © 2021 Tieu, Smith, Gopaluni, Kumararatne, McClure, Manson, Houghton and Jayne.
Conflict of interest statement
JT reports grants from Arthritis Australia (funded by Australian Rheumatology Association and Roche) and National Health and Medical Research Council during the conduct of this study. DK reports other support from CSL Behring, Shire/Takeda, Charities Fund Addenbrookes Hospital Cambridge and Grifols outside the submitted work, and membership of Immunoglobulin Demand Management Assessment Panel for National Health Service UK, membership of Clinical Reference Group for Immunology and Allergy National Health Service, England since 2019. AM reports personal fees and other support from CSL Behring, and other support from Takeda outside submitted work. DJ reports grants from Roche/Genentech during the conduct of the study, personal fees from Astra-Zeneca, Aurinia, and Boehringer, grants and personal fees from Chemocentryx, grants and personal fees from GSK, and grants from Sanofi outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past co-authorship with one of the authors, DJ.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

