Intestinal Microbiota-A Promising Target for Antiviral Therapy?
- PMID: 34054866
- PMCID: PMC8149780
- DOI: 10.3389/fimmu.2021.676232
Intestinal Microbiota-A Promising Target for Antiviral Therapy?
Abstract
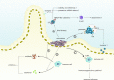
The intestinal microbiota is thought to be an important biological barrier against enteric pathogens. Its depletion, however, also has curative effects against some viral infections, suggesting that different components of the intestinal microbiota can play both promoting and inhibitory roles depending on the type of viral infection. The two primary mechanisms by which the microbiota facilitates or inhibits viral invasion involve participation in the innate and adaptive immune responses and direct or indirect interaction with the virus, during which the abundance and composition of the intestinal microbiota might be changed by the virus. Oral administration of probiotics, faecal microbiota transplantation (FMT), and antibiotics are major therapeutic strategies for regulating intestinal microbiota balance. However, these three methods have shown limited curative effects in clinical trials. Therefore, the intestinal microbiota might represent a new and promising supplementary antiviral therapeutic target, and more efficient and safer methods for regulating the microbiota require deeper investigation. This review summarizes the latest research on the relationship among the intestinal microbiota, anti-viral immunity and viruses and the most commonly used methods for regulating the intestinal microbiota with the goal of providing new insight into the antiviral effects of the gut microbiota.
Keywords: COVID-19; SARS-CoV-2; immunity; intestinal microbiota; virus.
Copyright © 2021 Yang, Yang, He, Zhu, Liu, Xu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

